Euryarchaeota is a phylum of archaea. Euryarchaeota are highly diverse and include methanogens, which produce methane and are often found in intestines; halobacteria, which survive extreme concentrations of salt; and some extremely thermophilic aerobes and anaerobes, which generally live at temperatures between 41 and 122 °C. They are separated from the other archaeans based mainly on rRNA sequences and their unique DNA polymerase.

Sulfolobus is a genus of microorganism in the family Sulfolobaceae. It belongs to the archaea domain.

Halobacteriales are an order of the Halobacteria, found in water saturated or nearly saturated with salt. They are also called halophiles, though this name is also used for other organisms which live in somewhat less concentrated salt water. They are common in most environments where large amounts of salt, moisture, and organic material are available. Large blooms appear reddish, from the pigment bacteriorhodopsin. This pigment is used to absorb light, which provides energy to create ATP. Halobacteria also possess a second pigment, halorhodopsin, which pumps in chloride ions in response to photons, creating a voltage gradient and assisting in the production of energy from light. The process is unrelated to other forms of photosynthesis involving electron transport; however, and halobacteria are incapable of fixing carbon from carbon dioxide.
Ignicoccus is a genus of hyperthermophillic Archaea living in marine hydrothermal vents. They were discovered in samples taken at the Kolbeinsey Ridge north of Iceland, as well as at the East Pacific Rise in 2000.
Pyrobaculum is a genus of the Thermoproteaceae.
In taxonomy, Thermococcus is a genus of thermophilic Archaea in the family the Thermococcaceae.

Methanobacterium is a genus of the Methanobacteriaceae family of Archaea. Despite the name, this genus belongs not to the bacterial domain but the archaeal domain. Methanobacterium are nonmotile and are anaerobic bacteria. They do not create endospores when nutrients are limited. Some members of this genus can use formate to reduce methane; others live exclusively through the reduction of carbon dioxide with hydrogen. They are ubiquitous in some hot, low-oxygen environments, such as anaerobic digestors, their waste water, and hot springs.
Thermococcus litoralis is a species of Archaea that is found around deep-sea hydrothermal vents as well as shallow submarine thermal springs and oil wells. It is an anaerobic organotroph hyperthermophile that is between 0.5–3.0 μm (20–118 μin) in diameter. Like the other species in the order thermococcales, T. litoralis is an irregular hyperthermophile coccus that grows between 55–100 °C (131–212 °F). Unlike many other thermococci, T. litoralis is non-motile. Its cell wall consists only of a single S-layer that does not form hexagonal lattices. Additionally, while many thermococcales obligately use sulfur as an electron acceptor in metabolism, T. litoralis only needs sulfur to help stimulate growth, and can live without it. T. litoralis has recently been popularized by the scientific community for its ability to produce an alternative DNA polymerase to the commonly used Taq polymerase. The T. litoralis polymerase, dubbed the vent polymerase, has been shown to have a lower error rate than Taq but due to its proofreading 3’–5’ exonuclease abilities.

The Nitrososphaerota are a phylum of the Archaea proposed in 2008 after the genome of Cenarchaeum symbiosum was sequenced and found to differ significantly from other members of the hyperthermophilic phylum Thermoproteota. Three described species in addition to C. symbiosum are Nitrosopumilus maritimus, Nitrososphaera viennensis, and Nitrososphaera gargensis. The phylum was proposed in 2008 based on phylogenetic data, such as the sequences of these organisms' ribosomal RNA genes, and the presence of a form of type I topoisomerase that was previously thought to be unique to the eukaryotes. This assignment was confirmed by further analysis published in 2010 that examined the genomes of the ammonia-oxidizing archaea Nitrosopumilus maritimus and Nitrososphaera gargensis, concluding that these species form a distinct lineage that includes Cenarchaeum symbiosum. The lipid crenarchaeol has been found only in Nitrososphaerota, making it a potential biomarker for the phylum. Most organisms of this lineage thus far identified are chemolithoautotrophic ammonia-oxidizers and may play important roles in biogeochemical cycles, such as the nitrogen cycle and the carbon cycle. Metagenomic sequencing indicates that they constitute ~1% of the sea surface metagenome across many sites.
Thermococcus celer is a Gram-negative, spherical-shaped archaeon of the genus Thermococcus. The discovery of T. celer played an important role in rerooting the tree of life when T. celer was found to be more closely related to methanogenic Archaea than to other phenotypically similar thermophilic species. T. celer was the first archaeon discovered to house a circularized genome. Several type strains of T. celer have been identified: Vu13, ATCC 35543, and DSM 2476.
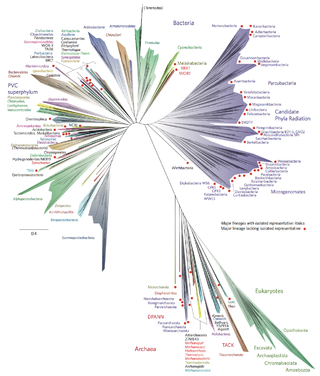
Bacterial phyla constitute the major lineages of the domain Bacteria. While the exact definition of a bacterial phylum is debated, a popular definition is that a bacterial phylum is a monophyletic lineage of bacteria whose 16S rRNA genes share a pairwise sequence identity of ~75% or less with those of the members of other bacterial phyla.
Thermococcus kodakarensis is a species of thermophilic archaea. The type strain T. kodakarensis KOD1 is one of the best-studied members of the genus.
Sulfolobus metallicus is a coccoid shaped thermophilic archaeon. It is a strict chemolithoautotroph gaining energy by oxidation of sulphur and sulphidic ores into sulfuric acid. Its type strain is Kra 23. It has many uses that take advantage of its ability to grow on metal media under acidic and hot environments.
Haladaptatus paucihalophilus is a halophilic archaeal species, originally isolated from a spring in Oklahoma. It uses a new pathway to synthesize glycine, and contains unique physiological features for osmoadaptation.
Acidilobus saccharovorans is a thermoacidophilic species of anaerobic archaea. The species was originally described in 2009 after being isolated from hot springs in Kamchatka.

DPANN is a superphylum of Archaea first proposed in 2013. Many members show novel signs of horizontal gene transfer from other domains of life. They are known as nanoarchaea or ultra-small archaea due to their smaller size (nanometric) compared to other archaea.
Haloferax sulfurifontis is a species of archaea in the family Haloferacaceae.
Metallosphaera hakonensis is a gram-negative, thermoacidophilic archaea discovered in the hot springs of Hakone National Park, Kanagawa, Japan.

Microbial oxidation of sulfur is the oxidation of sulfur by microorganisms to build their structural components. The oxidation of inorganic compounds is the strategy primarily used by chemolithotrophic microorganisms to obtain energy to survive, grow and reproduce. Some inorganic forms of reduced sulfur, mainly sulfide (H2S/HS−) and elemental sulfur (S0), can be oxidized by chemolithotrophic sulfur-oxidizing prokaryotes, usually coupled to the reduction of oxygen (O2) or nitrate (NO3−). Anaerobic sulfur oxidizers include photolithoautotrophs that obtain their energy from sunlight, hydrogen from sulfide, and carbon from carbon dioxide (CO2).
Blastopirellula marina, a member of the phylum Planctomycetota, is a halotolerant bacterium inhabiting aquatic environments. B. marina was determined to be a new species by utilizing 16s rRNA sequence analysis.