
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation with their smell.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.

Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane.

Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.
Cyclohexa-1,3-diene (also known as Benzane) is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). A naturally occurring derivative of cyclohexa-1,3-diene is terpinene, a component of pine oil.

Chromium(III) chloride (also called chromic chloride) describes any of several chemical compounds with the formula CrCl3 · xH2O, where x can be 0, 5, and 6. The anhydrous compound with the formula CrCl3 is a violet solid. The most common form of the trichloride is the dark green hexahydrate, CrCl3 · 6 H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Biphenyl is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one hydrogen may use the prefixes xenyl or diphenylyl.
An alkyne trimerisation is a [2+2+2] cycloaddition reaction in which three alkyne units react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthesis. Being a cycloaddition reaction, it has high atom economy. Many variations have been developed, including cyclisation of mixtures of alkynes and alkenes as well as alkynes and nitriles.

Prismane or 'Ladenburg benzene' is a polycyclic hydrocarbon with the formula C6H6. It is an isomer of benzene, specifically a valence isomer. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule are arranged in the shape of a six-atom triangular prism—this compound is the parent and simplest member of the prismanes class of molecules. Albert Ladenburg proposed this structure for the compound now known as benzene. The compound was not synthesized until 1973.

Dicobalt octacarbonyl is an organocobalt compound with composition Co2(CO)8. This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent member of a family of hydroformylation catalysts. Each molecule consists of two cobalt atoms bound to eight carbon monoxide ligands, although multiple structural isomers are known. Some of the carbonyl ligands are labile.
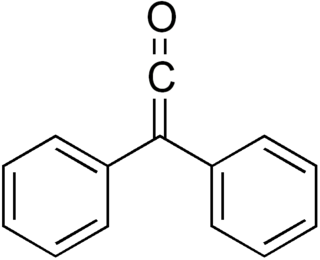
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumulene. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.
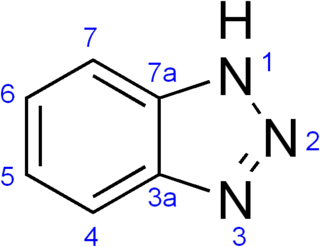
Benzotriazole (BTA) is a heterocyclic compound with the chemical formula C6H5N3. Its five-membered ring contains three consecutive nitrogen atoms. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and triazole. This white-to-light tan solid has a variety of uses, for instance, as a corrosion inhibitor for copper.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.
Iodane generally refers to any organic derivative of iodine. Without modifier, iodane is the systematic name for the parent hydride of iodine, HI. Thus, any organoiodine compound with general formula RI is a substituted iodane. However, as used in the context of organic synthesis, the term iodane more specifically refers to organoiodine compounds with nonstandard bond order of bonds between iodine and other atoms, i.e., bond order of iodine greater than 1, making this term a synonym for hypervalent iodine. These iodine compounds are hypervalent because the iodine atom formally contains more than the 8 electrons in the valence shell required for the octet rule. When iodine is ligated to an organic residue and electronegative ligands, hypervalent iodine occurs in a +3 oxidation state as iodine(III) or λ3-iodane, or in a +5 oxidation state as iodine(V) or λ5-iodane, or in a +7 oxidation state as iodine(VII) or λ7-iodane. Here, lambda convention is used to give the nonstandard bond order.

Indole is an aromatic, heterocyclic, organic compound with the formula C8H7N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin.
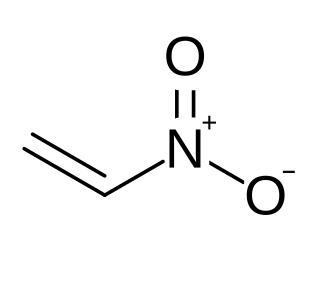
Nitroethylene (also known as nitroethene) is a liquid organic compound with the formula C2H3NO2. It is the simplest nitroalkene, which are unsaturated carbon chains with at least one double bond and a NO2 functional group. Nitroethylene serves as a useful intermediate in the production of various other chemicals.
Fétizon oxidation is the oxidation of primary and secondary alcohols utilizing the compound silver(I) carbonate absorbed onto the surface of celite also known as Fétizon's reagent first employed by Marcel Fétizon in 1968. It is a mild reagent, suitable for both acid and base sensitive compounds. Its great reactivity with lactols makes the Fétizon oxidation a useful method to obtain lactones from a diol. The reaction is inhibited significantly by polar groups within the reaction system as well as steric hindrance of the α-hydrogen of the alcohol.
















