In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood.
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of, pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.
In organic chemistry, an alkyl substituent is an alkane missing one hydrogen. The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of CnH2n+1. A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula CnH2n-1. Typically an alkyl is a part of a larger molecule. In structural formula, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula CH3−.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.

Nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound (with certain covalent characters), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents (83.05 g/100 mL of water at 20 °C) and its hygroscopic properties.
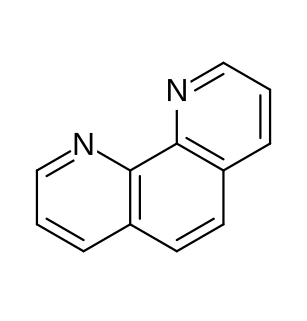
Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. It is used as a ligand in coordination chemistry, forming strong complexes with most metal ions. It is often sold as the monohydrate.

Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide ((C2H5)2SnI2), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn-C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.

Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.

A sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively charged ion (a "cation") featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula [SR3]+. Together with a negatively charged counterion, they give sulfonium salts. They are typically colorless solids that are soluble in organic solvent.
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives (for example Cn(CH3)n) and heterocyclic derivatives (for example BCnHn+1). Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.
Organovanadium chemistry is the chemistry of organometallic compounds containing a carbon to vanadium (V) chemical bond. Organovanadium compounds find only minor use as reagents in organic synthesis but are significant for polymer chemistry as catalysts.

Ruthenium(III) acetylacetonate is a coordination complex with the formula Ru(O2C5H7)3. O2C5H7− is the ligand called acetylacetonate. This compound exists as a dark violet solid that is soluble in most organic solvents. It is used as a precursor to other compounds of ruthenium.

2-Phenylpyridine is an organic compound with the formula C6H5C5H4N (or C11H9N). It is a colourless viscous liquid. The compound and related derivatives have attracted interest as precursors to highly fluorescent metal complexes of possible value as organic light emitting diodes (OLEDs).
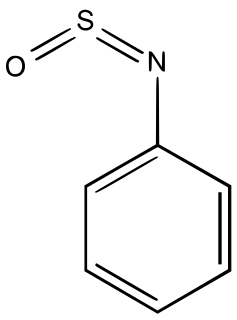
Sulfinylamines are organosulfur compounds with the formula RNSO where R = an organic substituent. These compounds are, formally speaking, derivatives of HN=S=O, i.e. analogues of sulfur dioxide and of sulfur diimide. A common example is N-sulfinylaniline. Sulfinyl amines are dienophile. They undergo [2+2] cycloaddition to ketenes.
2-Methyldodecane, an organic compound with a chemical formula C13H28, is an isomer of tridecane. It is produced by the reaction of 1-bromodecane and diisopropyl zinc. Reaction of decylmagnesium bromide and 2-bromopropane produce 2-methyldodecane too. Another method to produce 2-methyldodecane is react 1-dodecene and trimethylaluminium.












