
The Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 September 1987, and entered into force on 1 January 1989. Since then, it has undergone nine revisions, in 1990 (London), 1991 (Nairobi), 1992 (Copenhagen), 1993 (Bangkok), 1995 (Vienna), 1997 (Montreal), 1998 (Australia), 1999 (Beijing) and 2016 (Kigali) As a result of the international agreement, the ozone hole in Antarctica is slowly recovering. Climate projections indicate that the ozone layer will return to 1980 levels between 2040 and 2066. Due to its widespread adoption and implementation, it has been hailed as an example of successful international co-operation. Former UN Secretary-General Kofi Annan stated that "perhaps the single most successful international agreement to date has been the Montreal Protocol". In comparison, effective burden-sharing and solution proposals mitigating regional conflicts of interest have been among the success factors for the ozone depletion challenge, where global regulation based on the Kyoto Protocol has failed to do so. In this case of the ozone depletion challenge, there was global regulation already being installed before a scientific consensus was established. Also, overall public opinion was convinced of possible imminent risks.

The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.

Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.
Bromotrifluoromethane, commonly referred to by the code numbers Halon 1301, R13B1, Halon 13B1 or BTM, is an organic halide with the chemical formula CBrF3. It is used for gaseous fire suppression as a far less toxic alternative to bromochloromethane.

A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated due to their toxicity, flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change.

Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of a carboxylate ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other compounds of commercial interest.
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides.
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22, or CHClF
2. It was commonly used as a propellant and refrigerant. These applications were phased out under the Montreal Protocol in developed countries in 2020 due to the compound's ozone depletion potential (ODP) and high global warming potential (GWP), and in developing countries this process will be completed by 2030. R-22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene.
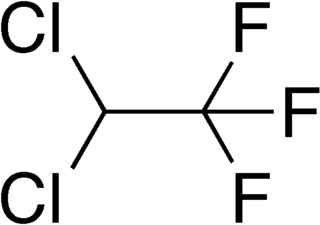
2,2-Dichloro-1,1,1-trifluoroethane or HCFC-123 is considered as an alternative to CFC-11 in low pressure refrigeration and HVAC systems, and should not be used in foam blowing processes or solvent applications. It is also the primary component of the Halotron I fire-extinguishing mixture.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota.

Rigid panel insulation, also referred to as continuous insulation, can be made from foam plastics such as polyurethane (PUR), polyisocyanurate (PIR), and polystyrene, or from fibrous materials such as fiberglass, rock and slag wool. Rigid panel continuous insulation is often used to provide a thermal break in the building envelope, thus reducing thermal bridging.
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula Cl2FC−CClF2. This colorless, volatile liquid is a versatile solvent.
Hydrofluoroethers (HFE) are a class of organic solvents. As non-ozone-depleting chemicals, they were developed originally as a replacement for CFCs, HFCs, HCFCs, and PFCs. They are typically colorless, odorless, tasteless, low toxicity, low viscosity, and liquid at room temperature. The boiling point of HFEs vary from 50 °C to nearly 100 °C. Although 3M first developed HFEs, other manufacturers have begun producing them.

1,1-Dichloro-1-fluoroethane is a haloalkane with the formula C
2H
3Cl
2F. It is one of the three isomers of dichlorofluoroethane. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment.

1-Chloro-1,1-difluoroethane (HCFC-142b) is a haloalkane with the chemical formula CH3CClF2. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment. It is primarily used as a refrigerant where it is also known as R-142b and by trade names including Freon-142b.
Stephen Oliver Andersen is the Director of Research at the Institute for Governance & Sustainable Development (IGSD) and former co-chair (1989–2012) of the Montreal Protocol Technology and Economic Assessment Panel (TEAP) where he also chaired and co-chaired Technical Options Committees, Task Forces and Special Reports. He is one of the founders and leading figures in the success of the Montreal Protocol on Substances that Deplete the Ozone Layer that has phased out the chemicals that deplete the stratospheric ozone that protects the Earth against the harmful effects of ultraviolet radiation that causes skin cancer, cataracts, and suppression of the human immune system, destroys agricultural crops and natural ecosystems and deteriorates the built environment. Because ozone-depleting chemicals are also powerful greenhouse gases the Montreal Protocol also protected climate. Dr. Andersen was instrumental in the 2016 Kigali Amendment that will phase down hydrofluorocarbons once necessary to phase out chlorofluorocarbons (CFCs) fast enough to avoid ozone tipping points, but no longer necessary now that environmentally superior replacements are available or soon to be available. For his ambitious campaign saving the ozone layer, Dr. Andersen earned the 2021 Future of Life Award along with Joe Farman and Susan Solomon.
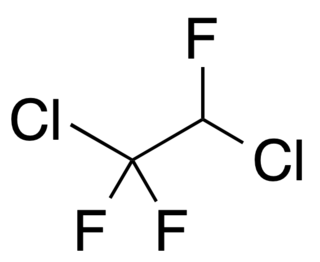
1,2-Dichloro-1,1,2-trifluoroethane is a volatile liquid chlorofluoroalkane composed of carbon, hydrogen, chlorine and fluorine, and with structural formula CClF2CHClF. It is also known as a refrigerant with the designation R-123a.

1,1-Dichloro-1,2-difluoroethane is a hydrochlorofluorocarbon. It is a volatile derivative of ethane. It appears as a colourless, odorless non-flammable liquid. The use of HCFC-132c is restricted by the US EPA through the Clean Air Act Amendments of 1990 which intend to phase-out the use of substances that deplete the ozone layer. HCFC-132c is cited as an ozone depleting substance; it is considered as a class II substance by the EPA.

Tetrachloro-1,1-difluoroethane or 1,1,1,2-tetrachloro-2,2-difluoroethane, Freon 112a, R-112a, or CFC-112a is an asymmetric chlorofluorocarbon isomer of tetrachloro-1,1-difluoroethane with formula CClF2CCl3. It contains ethane substituted by four chlorine atoms and two fluorine atoms. With a boiling point of 91.5°C it is the freon with second highest boiling point.













