In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. An group of the structure R2C=C=CR− is called allenyl, where R is H or some alkyl group. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding.
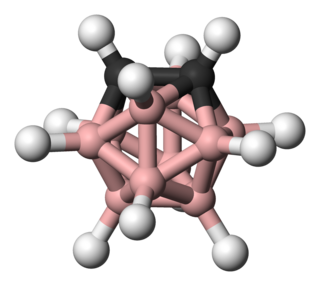
Cubane is a synthetic hydrocarbon compound with the formula C8H8, and that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.
Carboranes are electron-delocalized clusters composed of boron, carbon and hydrogen atoms. Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes.
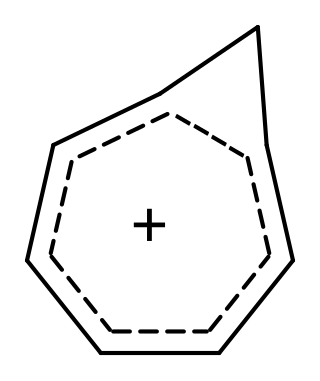
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.

Tetrathiafulvalene (TTF) is an organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.

Cyclopropanone is an organic compound with molecular formula (CH2)2CO consisting of a cyclopropane carbon framework with a ketone functional group. The parent compound is labile, being highly sensitive toward even weak nucleophiles. Surrogates of cyclopropanone include the ketals.

Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp2-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of aromatic organosulfur compounds. A few 1,2-dithietes have been isolated. 3,4-Bis(trifluoromethyl)-1,2-dithiete is a particularly stable example.

Dithietanes are saturated heterocyclic compounds that contain two divalent sulfur atoms and two sp3-hybridized carbon centers. Two isomers are possible for this class of organosulfur compounds:

In chemistry, dioxirane is a compound with formula CH
2O
2, whose molecule consists of a ring with one carbon and two oxygen atoms, and two hydrogen atoms attached to the carbon. It is a heterocyclic compound, the smallest cyclic organic peroxide.

Oxazoline is a five-membered heterocyclic organic compound with the formula C3H5NO. It is the parent of a family of compounds called oxazolines, which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the unsaturated analogues of oxazolidines, and they are isomeric with isoxazolines, where the N and O are directly bonded. Two isomers of oxazoline are known, depending on the location of the double bond.

In chemistry, the term pseudo-oxocarbon anion is used to refer to a negative ion that is conceptually derived from an oxocarbon anion through replacement of one or more of the basic oxygen atoms by chemically similar elements or functional groups, such as sulfur (S), selenium (Se), or dicyanomethylene (=C(CN)2).

In organic chemistry, a xylylene (sometimes quinone-dimethide) is any of the constitutional isomers having the formula C6H4(CH2)2. These compounds are related to the corresponding quinones and quinone methides by replacement of the oxygen atoms by CH2 groups. ortho- and para-xylylene are best known, although neither is stable in solid or liquid form. The meta form is a diradical. Certain substituted derivatives of xylylenes are however highly stable, such as tetracyanoquinodimethane and the xylylene dichlorides.

1,2-Dimethyldiborane is an organoboron compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is the dimer of methylborane, CH3BH2, the simplest alkylborane. 1,2-Dimethyldiborane can exist in a cis- and a trans arrangement. 1,2-Dimethyldiborane is an easily condensed, colorless gas that ignites spontaneously in air.
In chemistry, dithiadiazoles are a family of heterocyclic compounds with the formula RCN2S2. Two isomers have been studied: the 1,2‑dithia-3,5‑diazoles, in which the sulfur atoms are bonded to each other across the ring from the carbon atom, and the 1,3‑dithia-2,5‑diazoles, in which nitrogen and sulfur atoms alternate around the ring. In both cases, the neutral species are radicals that are of interest as examples of paramagnetic heterocycles. They have also attracted interest because of the tendency of the neutral species to form linear chain compounds, a theme in molecular electronics.

1,3-Diphenylisobenzofuran is a highly reactive diene that can scavenge unstable and short-lived dienophiles in a Diels-Alder reaction. It is furthermore used as a standard reagent for the determination of singlet oxygen, even in biological systems. Cycloadditions with 1,3-diphenylisobenzofuran and subsequent oxygen cleavage provide access to a variety of polyaromatics.

In organoboron chemistry, a carboryne is an unstable derivative of ortho-carborane with the formula B10C2H10. They are also called 1,2-dehydro-o-carboranes. The hydrogen atoms on the C2 unit in the parent o-carborane are missing. The compound resembles and is isolobal with benzyne. A carboryne compound was first generated in 1990 starting from o-carborane. The hydrogen atoms connected to carbon are removed by n-butyllithium in tetrahydrofuran and the resulting lithium dianion is reacted with bromine at 0 °C to form the bromo monoanion.

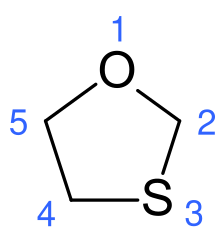
1,2-Dioxolane is a chemical compound with formula C3H6O2, consisting of a ring of three carbon atoms and two oxygen atoms in adjacent positions. Its condensed structural formula is [–(CH
2)3–O–O–].