
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry.

1,4-Butanediol, also called Butane-1,4-diol (other names include 1,4-B, BD, BDO and 1,4-BD), is a primary alcohol and an organic compound with the formula HOCH2CH2CH2CH2OH. It is a colorless viscous liquid first synthesized in 1890 via acidic hydrolysis of N,N'-dinitro-1,4-butanediamine by Dutch chemist Pieter Johannes Dekkers, who called it "tetramethylene glycol".
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound with the formula SnCl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds. It was first discovered by Andreas Libavius (1550–1616) and was known as spiritus fumans libavii.

Thiourea is an organosulfur compound with the formula SC(NH2)2 and the structure H2N−C(=S)−NH2. It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom ; however, the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. Thioureas are a broad class of compounds with the general structure R2N−C(=S)−NR2.

Gamma-butyrolactone (GBL) or γ-butyrolactone is a hygroscopic, colorless, water-miscible liquid with a weak, characteristic odor. It is the simplest 4-carbon lactone. It is mainly used as an intermediate in the production of other chemicals, such as N-methyl-2-pyrrolidone.
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of precursors to nylon.

n-Butyllithium C4H9Li (abbreviated n-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly employed as a strong base (superbase) in the synthesis of organic compounds as in the pharmaceutical industry.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
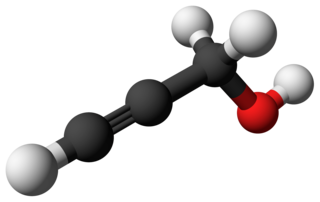
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents.
In organic chemistry, the Paal–Knorr synthesis is a reaction used to synthesize substituted furans, pyrroles, or thiophenes from 1,4-diketones. It is a synthetically valuable method for obtaining substituted furans and pyrroles, which are common structural components of many natural products. It was initially reported independently by German chemists Carl Paal and Ludwig Knorr in 1884 as a method for the preparation of furans, and has been adapted for pyrroles and thiophenes. Although the Paal–Knorr synthesis has seen widespread use, the mechanism wasn't fully understood until it was elucidated by V. Amarnath et al. in the 1990s.

4-Nitroaniline, p-nitroaniline or 1-amino-4-nitrobenzene is an organic compound with the formula C6H6N2O2. A yellow solid, it is one of three isomers of nitroaniline. It is an intermediate in the production of dyes, antioxidants, pharmaceuticals, gasoline, gum inhibitors, poultry medicines, and as a corrosion inhibitor.

Pinacolone (3,3-dimethyl-2-butanone) is an important ketone in organic chemistry. It is a colorless liquid and has a slight peppermint- or camphor- odor. It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadimefon and in synthesis of the herbicide metribuzin. The molecule is an unsymmetrical ketone. The α-methyl group can participate in condensation reactions. The carbonyl group can undergo the usual reactions. It is a Schedule 3 compound under the Chemical Weapons Convention 1993, due to being related to pinacolyl alcohol, which is used in the production of soman. It is also a controlled export in Australia Group member states.
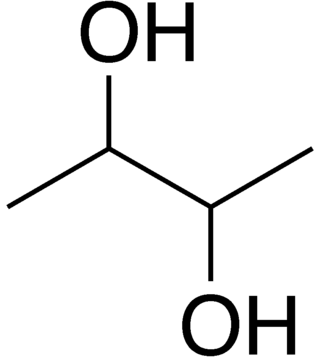
2,3-Butanediol is the organic compound with the formula (CH3CHOH)2. It is classified as a vic-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids. Applications include precursors to various plastics and pesticides.
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
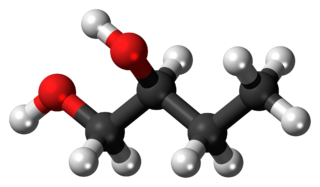
1,2-Butanediol is the organic compound with the formula HOCH2(HO)CHCH2CH3. It is classified as a vic-diol (glycol). It is chiral, although typically it is encountered as the racemic mixture. It is a colorless liquid.

Endiandric acid C, isolated from the tree Endiandra introrsa, is a well characterized chemical compound. Endiadric acid C is reported to have better antibiotic activity than ampicillin.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.
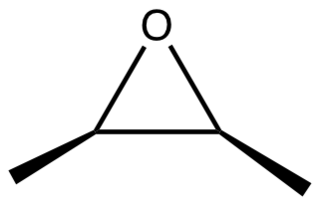
2,3-Epoxybutane is an organic compound with the formula CH3CH(O)CHCH3. It is an epoxide. The compound exists as three stereoisomers, a pair of enantiomers and the meso isomer. All are colorless liquids.




















