
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resultant aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and pharmaceuticals. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry.
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of precursors to nylon.
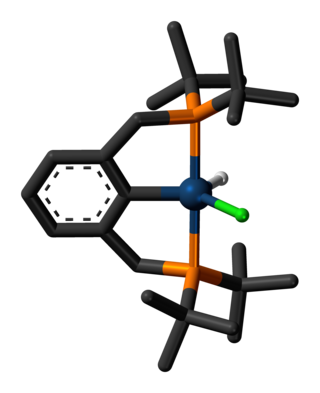
In chemistry, a transition metal pincer complex is a type of coordination complex with a pincer ligand. Pincer ligands are chelating agents that binds tightly to three adjacent coplanar sites in a meridional configuration. The inflexibility of the pincer-metal interaction confers high thermal stability to the resulting complexes. This stability is in part ascribed to the constrained geometry of the pincer, which inhibits cyclometallation of the organic substituents on the donor sites at each end. In the absence of this effect, cyclometallation is often a significant deactivation process for complexes, in particular limiting their ability to effect C-H bond activation. The organic substituents also define a hydrophobic pocket around the reactive coordination site. Stoichiometric and catalytic applications of pincer complexes have been studied at an accelerating pace since the mid-1970s. Most pincer ligands contain phosphines. Reactions of metal-pincer complexes are localized at three sites perpendicular to the plane of the pincer ligand, although in some cases one arm is hemi-labile and an additional coordination site is generated transiently. Early examples of pincer ligands were anionic with a carbanion as the central donor site and flanking phosphine donors; these compounds are referred to as PCP pincers.
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (Ph2PCH2)2 (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.

In coordination chemistry, the first coordination sphere refers to the array of molecules and ions directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ways to the first coordination sphere.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).
A hydrogenase mimic or bio-mimetic is an enzyme mimic of hydrogenases.

Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.

1,1-Bis(diphenylphosphino)methane (dppm), is an organophosphorus compound with the formula CH2(PPh2)2. Dppm, a white, crystalline powder, is used in inorganic and organometallic chemistry as a ligand. It is more specifically a chelating ligand because it is a ligand that can bond to metals with two phosphorus donor atoms. The natural bite angle is 73°.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).
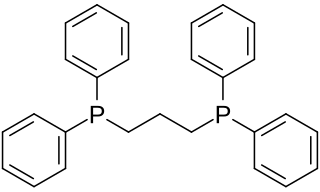
1,3-Bis(diphenylphosphino)propane (dppp) is an organophosphorus compound with the formula Ph2P(CH2)3PPh2. The compound is a white solid that is soluble in organic solvents. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is classified as a diphosphine ligand in coordination chemistry and homogeneous catalysis.
Hydroacylation is a type of organic reaction in which an alkene is inserted into the a formyl C-H bond. The product is a ketone. The reaction requires a metal catalyst. It is almost invariably practiced as an intramolecular reaction using homogeneous catalysts, often based on rhodium phosphines.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).
Jenny Yue-fon Yang is an American chemist. She is a Professor of chemistry at the University of California, Irvine where she leads a research group focused on inorganic chemistry, catalysis, and solar fuels.
Mary Rakowski DuBois is an inorganic chemist, now retired from Pacific Northwest National Laboratory (PNNL). She made multiple contributions to inorganic and organometallic chemistry, focusing on synthetic and mechanistic studies. In recognition of her scientific contributions, she received several awards.
Hydroxymethylation is a chemical reaction that installs the CH2OH group. The transformation can be implemented in many ways and applies to both industrial and biochemical processes.










