The cumene process is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene, the intermediate material during the process. It was invented by R. Ūdris and P. Sergeyev in 1942 (USSR), and independently by Heinrich Hock in 1944.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Pentachlorophenol (PCP) is an organochlorine compound used as a pesticide and a disinfectant. First produced in the 1930s, it is marketed under many trade names. It can be found as pure PCP, or as the sodium salt of PCP, the latter of which dissolves easily in water. It can be biodegraded by some bacteria, including Sphingobium chlorophenolicum.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups at various positions relative to the hydroxyl group. Six isomers exist, of which 2,6-xylenol with both methyl groups in an ortho position with respect to the hydroxyl group is the most important. The name xylenol is a portmanteau of the words xylene and phenol.
2-Chlorophenol or ortho-chlorophenol is an organic compound with the formula C6H4ClOH. It is one of three isomeric monochloride derivatives of phenol. As from occasional use as a disinfectant, it has few applications. It is an intermediate in the polychlorination of phenol. 2-Chlorophenol is a colorless liquid, although commercial samples are often yellow or amber-colored. It has an unpleasant, penetrating (carbolic) odor. It is poorly soluble in water.
A chlorophenol is any organochloride of phenol that contains one or more covalently bonded chlorine atoms. There are five basic types of chlorophenols and 19 different chlorophenols in total when positional isomerism is taken into account. Chlorophenols are produced by electrophilic halogenation of phenol with chlorine.

Naphthalenesulfonates are derivatives of sulfonic acid which contain a naphthalene functional unit. A related family of compounds are the aminonaphthalenesulfonic acids. Of commercial importance are the alkylnaphthalene sulfonates, which are used as superplasticizers in concrete. They are produced on a large scale by condensation of naphthalenesulfonate or alkylnaphthalenesulfonates with formaldehyde.
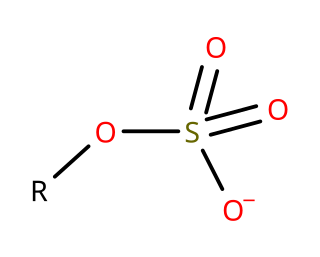
In organosulfur chemistry, organosulfates are a class of organic compounds sharing a common functional group with the structure R−O−SO−3. The SO4 core is a sulfate group and the R group is any organic residue. All organosulfates are formally esters derived from alcohols and sulfuric acid although many are not prepared in this way. Many sulfate esters are used in detergents, and some are useful reagents. Alkyl sulfates consist of a hydrophobic hydrocarbon chain, a polar sulfate group and either a cation or amine to neutralize the sulfate group. Examples include: sodium lauryl sulfate and related potassium and ammonium salts.

2,4,6-Trichlorophenol, also known as TCP, phenaclor, Dowicide 2S, Dowcide 2S, omal, is a chlorinated phenol that has been used as a fungicide, herbicide, insecticide, antiseptic, defoliant, and glue preservative. It is a clear to yellowish crystalline solid with a strong, phenolic odor. It decomposes on heating to produce toxic and corrosive fumes including hydrogen chloride and chlorine.
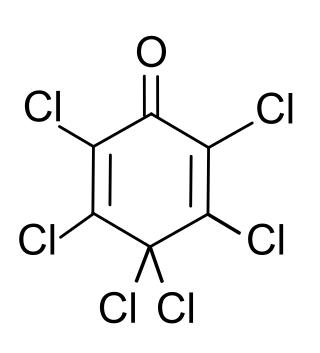
Hexachlorocyclohexa-2,5-dien-1-one, sometimes informally called hexachlorophenol (HCP) is an organochlorine compound. It can be prepared from phenol. Despite the informal name, the compound is not a phenol but is a ketone. The informal name is derived from its method of preparation which includes phenol as a reagent.
2,4-Dichlorophenol (2,4-DCP) is a chlorinated derivative of phenol with the molecular formula Cl2C6H3OH. It is a white solid that is mildly acidic (pKa = 7.9). It is produced on a large scale as a precursor to the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D).
Dichlorophenols (DCPs) are any of several chemical compounds which are derivatives of phenol containing two chlorine atoms. There are six isomers:

Benzenesulfonic acid (conjugate base benzenesulfonate) is an organosulfur compound with the formula C6H6O3S. It is the simplest aromatic sulfonic acid. It forms white deliquescent sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in benzene and insoluble in nonpolar solvents like diethyl ether. It is often stored in the form of alkali metal salts. Its aqueous solution is strongly acidic.

Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule that functions as a mild oxidant.
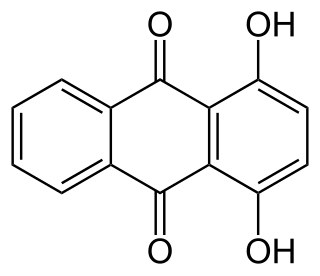
1,4-Dihydroxyanthraquinone, also called quinizarin or Solvent Orange 86, is an organic compound derived from anthroquinone. Quinizarin is an orange or red-brown crystalline powder. It is formally derived from anthraquinone by replacement of two hydrogen atoms by hydroxyl (OH) groups. It is one of ten dihydroxyanthraquinone isomers and occurs in small amounts in the root of the madder plant, Rubia tinctorum.
4-Nitrotoluene or para-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is a pale yellow solid. It is one of three isomers of nitrotoluene.

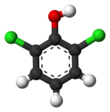
3-Chlorophenol is an organic compound with the molecular formula C6H4ClOH. It is one of three isomers of monochlorophenol. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Together with 3,5-dichlorophenol, it is prepared industrially by dechlorination of polychlorophenols. Alternatively, it arises via the cumene process, which starts with the alkylation of chlorobenzene with propylene.

In organic chemistry, the desulfonation reaction is the hydrolysis of sulfonic acids:
4-Chlorophenol is an organic compound with the formula C6H4ClOH. It is one of three monochlorophenol isomers. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Its pKa is 9.14.