
In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".
The biophilia hypothesis suggests that humans possess an innate tendency to seek connections with nature and other forms of life. Edward O. Wilson introduced and popularized the hypothesis in his book, Biophilia (1984). He defines biophilia as "the urge to affiliate with other forms of life".
The Kerr–Newman metric is the most general asymptotically flat, stationary solution of the Einstein–Maxwell equations in general relativity that describes the spacetime geometry in the region surrounding an electrically charged, rotating mass. It generalizes the Kerr metric by taking into account the field energy of an electromagnetic field, in addition to describing rotation. It is one of a large number of various different electrovacuum solutions, that is, of solutions to the Einstein–Maxwell equations which account for the field energy of an electromagnetic field. Such solutions do not include any electric charges other than that associated with the gravitational field, and are thus termed vacuum solutions.

Ezra Theodore Newman was an American physicist, known for his many contributions to general relativity theory. He was professor emeritus at the University of Pittsburgh. Newman was awarded the 2011 Einstein Prize from the American Physical Society.
In organic chemistry, the Arndt–Eistert reaction is the conversion of a carboxylic acid to its homologue. Named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978), the method entails treating an acid chlorides with diazomethane. It is a popular method of producing β-amino acids from α-amino acids.
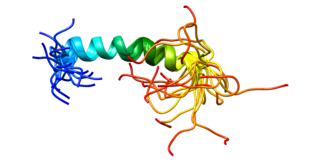
Platelet endothelial cell adhesion molecule (PECAM-1) also known as cluster of differentiation 31 (CD31) is a protein that in humans is encoded by the PECAM1 gene found on chromosome17q23.3. PECAM-1 plays a key role in removing aged neutrophils from the body.

C-C motif chemokine 11 also known as eosinophil chemotactic protein and eotaxin-1 is a protein that in humans is encoded by the CCL11 gene. This gene is encoded on three exons and is located on chromosome 17.

The northern or sandy nail-tail wallaby is a species of macropod found across northern Australia on arid and sparsely wooded plains. The largest species of the genus Onychogalea, it is a solitary and nocturnal herbivorous browser that selects its food from a wide variety of grasses and succulent plant material. Distinguished by a slender and long-limbed form that resembles the typical and well known kangaroos, although their standing height is shorter, around half of one metre, and their weight is less than nine kilograms. As with some medium to large kangaroo species, such as Osphranter rufus, they have an unusual pentapedal motion at slow speeds by stiffening the tail for a fifth limb. When fleeing a disturbance, they hop rapidly with the tail curled back and repeatedly utter the sound "wuluhwuluh". Their exceptionally long tail has a broad fingernail-like protuberance beneath a dark crest of hair at its end, a peculiarity of the genus that is much broader than the other species. The name unguifera, meaning claw, is a reference to this extraordinary attribute, the purpose of which is unknown.

C-C chemokine receptor type 3 is a protein that in humans is encoded by the CCR3 gene.
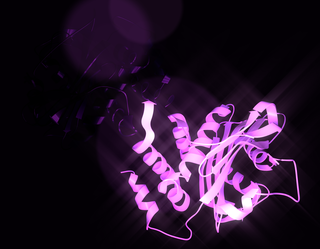
Electroneutral sodium bicarbonate exchanger 1 is a protein that in humans is encoded by the SLC4A8 gene.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
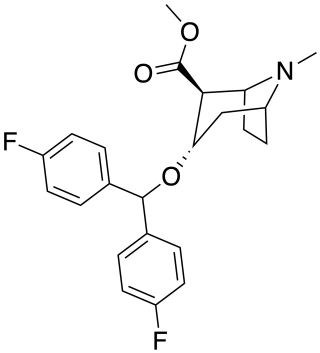
Difluoropine (O-620) is a stimulant drug synthesised from tropinone, which acts as a potent and selective dopamine reuptake inhibitor. Difluoropine is unique among the tropane-derived dopamine reuptake inhibitors in that the active stereoisomer is the (S) enantiomer rather than the (R) enantiomer, the opposite way round compared to natural cocaine. It is structurally related to benztropine and has similar anticholinergic and antihistamine effects in addition to its dopamine reuptake inhibitory action.
The phonology of the Zuni language as spoken in the southwestern United States is described here. Phonology is a branch of linguistics that studies how languages or dialects systematically organize their sounds.
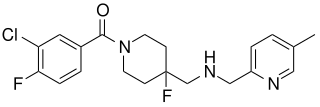
Befiradol is an experimental drug being studied for the treatment of levodopa-induced dyskinesia. It is a potent and selective 5-HT1A receptor full agonist.
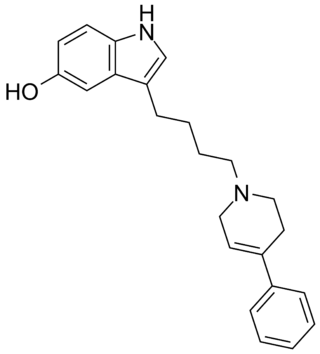
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.

JWH-007 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It was first reported in 1994 by a group including the noted cannabinoid chemist John W. Huffman. It was the most active of the first group of N-alkyl naphoylindoles discovered by the team led by John W Huffman, several years after the family was initially described with the discovery of the N-morpholinylethyl compounds pravadoline (WIN 48,098), JWH-200 (WIN 55,225) and WIN 55,212-2 by the Sterling Winthrop group. Several other N-alkyl substituents were found to be active by Huffman's team including the n-butyl, n-hexyl, 2-heptyl, and cyclohexylethyl groups, but it was subsequently determined that the 2-methyl group on the indole ring is not required for CB1 binding, and tends to increase affinity for CB2 instead. Consequently, the 2-desmethyl derivative of JWH-007, JWH-018, has slightly higher binding affinity for CB1, with an optimum binding of 9.00 nM at CB1 and 2.94 nM at CB2, and JWH-007 displayed optimum binding of 9.50 nM at CB1 and 2.94 nM at CB2.
The Newman–Kwart rearrangement is a type of rearrangement reaction in which the aryl group of an O-aryl thiocarbamate, ArOC(=S)NMe2, migrates from the oxygen atom to the sulfur atom, forming an S-aryl thiocarbamate, ArSC(=O)NMe2. The reaction is named after its discoverers, Melvin Spencer Newman and Harold Kwart. The reaction is a manifestation of the double bond rule. The Newman–Kwart reaction represents a useful synthetic tool for the preparation of thiophenol derivatives.

Dazoxiben is an orally active thromboxane synthase inhibitor. It has shown a significant clinical improvement in patients with Raynaud's syndrome.
The Louvain method for community detection is a method to extract non-overlapping communities from large networks created by Blondel et al. from the University of Louvain. The method is a greedy optimization method that appears to run in time where is the number of nodes in the network.

1-Naphthalenethiol is an organosulfur compound with the formula C10H7SH. It is a white solid. It is one of two monothiols of naphthalene, the other being 2-naphthalenethiol.
















