
Amygdalin is a naturally occurring chemical compound best known for being falsely promoted as a cancer cure. It is found in many plants, but most notably in the seeds (kernels) of apricots, bitter almonds, apples, peaches, and plums.

A cyanide is a chemical compound that contains the group C≡N. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Hydrogen cyanide (HCN), sometimes called prussic acid, is a chemical compound with the chemical formula HCN. It is a colorless, extremely poisonous and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valuable precursor to many chemical compounds ranging from polymers to pharmaceuticals.
Acetonitrile is the chemical compound with the formula CH
3CN. This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene.
Methylene blue, also known as methylthioninium chloride, is a medication and dye. As a medication, it is mainly used to treat methemoglobinemia. Specifically, it is used to treat methemoglobin levels that are greater than 30% or in which there are symptoms despite oxygen therapy. It has previously been used for cyanide poisoning and urinary tract infections, but this use is no longer recommended. It is typically given by injection into a vein.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons; to a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.
A nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates. Mercury(II) thiocyanate was formerly used in pyrotechnics.
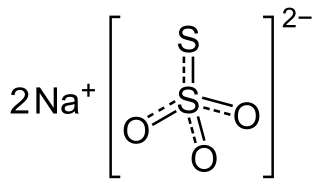
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula Na2S2O3.xH2O. Typically it is available as the white or colorless pentahydrate, Na2S2O3·5H2O. The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water.
Amatoxin is the collective name of a subgroup of at least eight related toxic compounds found in several genera of poisonous mushrooms, most notably the death cap and several other members of the genus Amanita, as well as some Conocybe, Galerina and Lepiota mushroom species. Amatoxins are lethal in even small doses, as little as half a mushroom. Unlike many ingested poisons, they cannot be destroyed by heat without destroying the mushrooms beyond edibility first, so cooking the poisonous mushrooms does not diminish their lethality.
Smoke inhalation is the primary cause of death for victims of fires. The inhalation or exposure to hot gaseous products of combustion can cause serious respiratory complications.

Thiosulfate is an oxyanion of sulfur.

Rhodanese, also known as rhodanase, thiosulfate sulfurtransferase, thiosulfate cyanide transsulfurase, and thiosulfate thiotransferase, is a mitochondrial enzyme that detoxifies cyanide (CN−) by converting it to thiocyanate (SCN−).
4-Dimethylaminophenol (DMAP) is an aromatic compound containing both phenol and amine functional groups. It has the molecular formula C8H11NO.
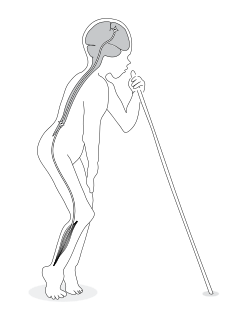
Konzo is an epidemic paralytic disease occurring among hunger-stricken rural populations in Africa where a diet dominated by insufficiently processed cassava results in simultaneous malnutrition and high dietary cyanide intake. Konzo was first described by Giovanni Trolli in 1938 who compiled the observations from eight doctors working in the Kwango area of the Belgian Congo.

Cyanide poisoning is poisoning that results from exposure to a number of forms of cyanide. Early symptoms include headache, dizziness, fast heart rate, shortness of breath, and vomiting. This may then be followed by seizures, slow heart rate, low blood pressure, loss of consciousness, and cardiac arrest. Onset of symptoms is usually within a few minutes. If a person survives, there may be long-term neurological problems.

In enzymology, a 3-mercaptopyruvate sulfurtransferase is an enzyme that catalyzes the chemical reactions of 3-mercaptopyruvate. This enzyme belongs to the family of transferases, specifically the sulfurtransferases. This enzyme participates in cysteine metabolism. It is encoded by the MPST gene.
In enzymology, a tRNA sulfurtransferase is an enzyme that catalyzes the chemical reaction
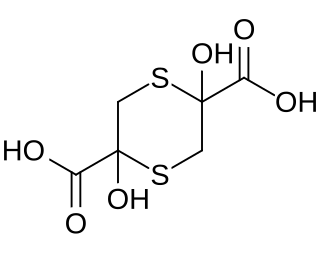
Sulfanegen is an experimental antidote for cyanide poisoning. It is being studied as a prodrug for 3-mercaptopyruvic acid (3-MP). 3-MP has been studied as a potential treatment for cyanide poisoning, but the half-life is too short for it to be clinically effective. Instead, alternative chemicals such as sulfanegen, the hemithioacetal cyclic dimer of 3-MP, are being evaluated that produce 3-MP in vivo to compensate for the short half-life of 3-MP itself.

Sodium thiosulfate, also spelled sodium thiosulphate, is used as a medication to treat cyanide poisoning, pityriasis versicolor, and to decrease side effects from cisplatin. For cyanide poisoning it is often used after the medication sodium nitrite and typically only recommended for severe cases. It is either given by injection into a vein or applied to the skin.










