
Vitamin B6 is one of the B vitamins, and thus an essential nutrient. The term refers to a group of six chemically similar compounds, i.e., "vitamers", which can be interconverted in biological systems. Its active form, pyridoxal 5′-phosphate, serves as a coenzyme in more than 140 enzyme reactions in amino acid, glucose, and lipid metabolism.

Aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme, located in region 7p12.2-p12.1.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.
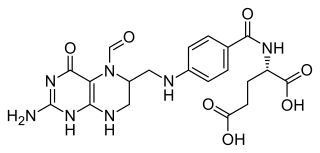
Folinic acid, also known as leucovorin, is a medication used to decrease the toxic effects of methotrexate and pyrimethamine. It is also used in combination with 5-fluorouracil to treat colorectal cancer and pancreatic cancer, may be used to treat folate deficiency that results in anemia, and methanol poisoning. It is taken by mouth, injection into a muscle, or injection into a vein.

Sphingosine kinase (SphK) is a conserved lipid kinase that catalyzes formation sphingosine-1-phosphate (S1P) from the precursor sphingolipid sphingosine. Sphingolipid metabolites, such as ceramide, sphingosine and sphingosine-1-phosphate, are lipid second messengers involved in diverse cellular processes. There are two forms of SphK, SphK1 and SphK2. SphK1 is found in the cytosol of eukaryotic cells, and migrates to the plasma membrane upon activation. SphK2 is localized to the nucleus.

Lipid signaling, broadly defined, refers to any biological cell signaling event involving a lipid messenger that binds a protein target, such as a receptor, kinase or phosphatase, which in turn mediate the effects of these lipids on specific cellular responses. Lipid signaling is thought to be qualitatively different from other classical signaling paradigms because lipids can freely diffuse through membranes. One consequence of this is that lipid messengers cannot be stored in vesicles prior to release and so are often biosynthesized "on demand" at their intended site of action. As such, many lipid signaling molecules cannot circulate freely in solution but, rather, exist bound to special carrier proteins in serum.
Sphingosine-1-phosphate (S1P) is a signaling sphingolipid, also known as lysosphingolipid. It is also referred to as a bioactive lipid mediator. Sphingolipids at large form a class of lipids characterized by a particular aliphatic aminoalcohol, which is sphingosine.

Pyridoxine 5′-phosphate oxidase is an enzyme, encoded by the PNPO gene, that catalyzes several reactions in the vitamin B6 metabolism pathway. Pyridoxine 5′-phosphate oxidase catalyzes the final, rate-limiting step in vitamin B6 metabolism, the biosynthesis of pyridoxal 5′-phosphate, the biologically active form of vitamin B6 which acts as an essential cofactor. Pyridoxine 5′-phosphate oxidase is a member of the enzyme class oxidases, or more specifically, oxidoreductases. These enzymes catalyze a simultaneous oxidation-reduction reaction. The substrate oxidase enzymes is hydroxlyated by one oxygen atom of molecular oxygen. Concurrently, the other oxygen atom is reduced to water. Even though molecular oxygen is the electron acceptor in these enzymes' reactions, they are unique because oxygen does not appear in the oxidized product.
Ceramidase is an enzyme which cleaves fatty acids from ceramide, producing sphingosine (SPH) which in turn is phosphorylated by a sphingosine kinase to form sphingosine-1-phosphate (S1P).
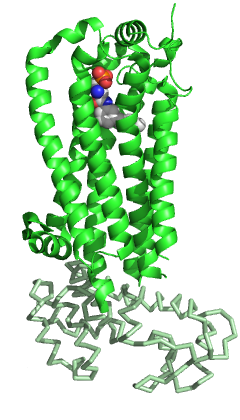
Sphingosine-1-phosphate receptor 1, also known as endothelial differentiation gene 1 (EDG1) is a protein that in humans is encoded by the S1PR1 gene. S1PR1 is a G-protein-coupled receptor which binds the bioactive signaling molecule sphingosine 1-phosphate (S1P). S1PR1 belongs to a sphingosine-1-phosphate receptor subfamily comprising five members (S1PR1-5). S1PR1 was originally identified as an abundant transcript in endothelial cells and it has an important role in regulating endothelial cell cytoskeletal structure, migration, capillary-like network formation and vascular maturation. In addition, S1PR1 signaling is important in the regulation of lymphocyte maturation, migration and trafficking.

Sphingosine-1-phosphate receptor 4 also known as S1PR4 is a human gene which encodes a G protein-coupled receptor which binds the lipid signaling molecule sphingosine 1-phosphate (S1P). Hence this receptor is also known as S1P4.

Sphingosine-1-phosphate receptor 2, also known as S1PR2 or S1P2, is a human gene which encodes a G protein-coupled receptor which binds the lipid signaling molecule sphingosine 1-phosphate (S1P).
The enzyme pyridoxal phosphatase (EC 3.1.3.74) catalyzes the reaction

In enzymology, a pyridoxine 5'-phosphate synthase (EC 2.6.99.2) is an enzyme that catalyzes the chemical reaction
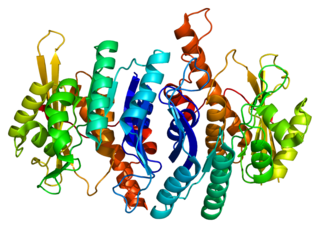
Pyridoxal kinase is an enzyme that in humans is encoded by the PDXK gene.
Vitamins occur in a variety of related forms known as vitamers. A vitamer of a particular vitamin is one of several related compounds that performs the functions of said vitamin and prevents the symptoms of deficiency of said vitamin.

Ginkgotoxin (4'-O-methylpyridoxine) is a neurotoxin naturally occurring in Ginkgo biloba. It is an antivitamin structurally related to vitamin B6 (pyridoxine). It has the capacity to induce epileptic seizures.

The sphingosine-1-phosphate receptors are a class of G protein-coupled receptors that are targets of the lipid signalling molecule Sphingosine-1-phosphate (S1P). They are divided into five subtypes: S1PR1, S1PR2, S1PR3, S1PR4 and S1PR5.

Sphingosine-1-phosphate receptor modulators are a class of drugs used as immunomodulators, most notably in cases of multiple sclerosis.
Megavitamin-B6 syndrome, also known as hypervitaminosis B6, vitamin B6 toxicity, and vitamin B6 excess, is a medical condition characterized by adverse effects resulting from excessive intake of vitamin B6. Primarily affecting the nervous system, this syndrome manifests through symptoms such as peripheral sensory neuropathy, characterized by numbness, tingling, and burning sensations in the limbs. The condition is usually triggered by chronic dietary supplementation of vitamin B6 but can also result from acute over-dosages, whether orally or parenterally.













