An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

Fischer esterification or Fischer–Speier esterification is a special type of esterification by refluxing a carboxylic acid and an alcohol in the presence of an acid catalyst. The reaction was first described by Emil Fischer and Arthur Speier in 1895. Most carboxylic acids are suitable for the reaction, but the alcohol should generally be primary or secondary. Tertiary alcohols are prone to elimination. Contrary to common misconception found in organic chemistry textbooks, phenols can also be esterified to give good to near quantitative yield of products. Commonly used catalysts for a Fischer esterification include sulfuric acid, p-toluenesulfonic acid, and Lewis acids such as scandium(III) triflate. For more valuable or sensitive substrates other, milder procedures such as Steglich esterification are used. The reaction is often carried out without a solvent or in a non-polar solvent to facilitate the Dean-Stark method. Typical reaction times vary from 1–10 hours at temperatures of 60-110 °C.
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
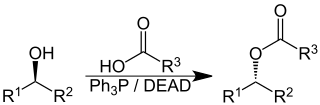
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). Typical protocol is to add the phosphine and azodicarboxylate together at -10C, typically in THF or toluene, until a white precipitate forms. This white, cloudy suspension is the ylide. Then a solution of the nucleophile and alcohol are added together and the reaction can, and in many cases is, heated to reflux. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion of stereochemistry in classic SN2 fashion as the nucleophile displaces it. A common side-product is produced when the azodicarboxylate displaces the leaving group instead of the desired nucleophile. This happens if the nucleophile is not acidic enough or is not nucleophilic enough due to steric or electronic constraints. A variation of this reaction utilizing a nitrogen nucleophile is known as a Fukuyama-Mitsunobu.

N,N′-Dicyclohexylcarbodiimide (DCC or DCCD) is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. Its primary use is to couple amino acids during artificial peptide synthesis. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water.
The Dakin–West reaction is a chemical reaction that transforms an amino-acid into a keto-amide using an acid anhydride and a base, typically pyridine. It is named for Henry Drysdale Dakin (1880–1952) and Randolph West (1890–1949). In 2016 Schreiner and coworkers reported the first asymmetric variant of this reaction employing short oligopeptides as catalysts.
Nucleophilic acyl substitution describe a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl derivative – such as an acid halide, anhydride, or ester. The resulting product is a carbonyl-containing compound in which the nucleophile has taken the place of the leaving group present in the original acyl derivative. Because acyl derivatives react with a wide variety of nucleophiles, and because the product can depend on the particular type of acyl derivative and nucleophile involved, nucleophilic acyl substitution reactions can be used to synthesize a variety of different products.

The Nicolaou Taxol total synthesis, published by K. C. Nicolaou and his group in 1994 concerns the total synthesis of Taxol. Taxol is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the pacific yew.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. This enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in reactivity between enantiomers or enantiomeric complexes. Kinetic resolution is a concept in organic chemistry and can be used for the preparation of chiral molecules in organic synthesis. Kinetic resolution reactions utilizing purely synthetic reagents and catalysts are much less common than the use of enzymatic kinetic resolution in application towards organic synthesis, although a number of useful synthetic techniques have been developed in the past 30 years.
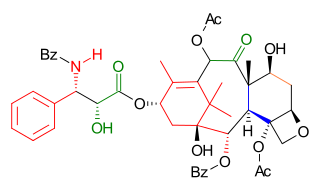
The Wender Taxol total synthesis in organic chemistry describes a Taxol total synthesis by the group of Paul Wender at Stanford University published in 1997. This synthesis has much in common with the Holton Taxol total synthesis in that it is a linear synthesis starting from a naturally occurring compound with ring construction in the order A,B,C,D. The Wender effort is shorter by approximately 10 steps.
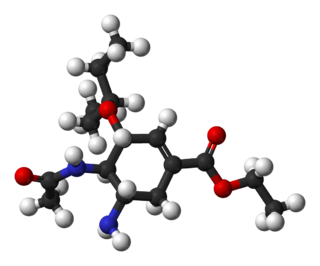
Oseltamivir total synthesis concerns the total synthesis of the antiinfluenza drug oseltamivir marketed by Hoffmann-La Roche under the trade name Tamiflu. Its commercial production starts from the biomolecule shikimic acid harvested from Chinese star anise and from recombinant E. coli. Control of stereochemistry is important: the molecule has three stereocenters and the sought-after isomer is only 1 of 8 stereoisomers.

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.
The Yamaguchi esterification is the chemical reaction of an aliphatic carboxylic acid and 2,4,6-trichlorobenzoyl chloride to form a mixed anhydride which, upon reaction with an alcohol in the presence of stoichiometric amount of DMAP, produces the desired ester. It was first reported by Masaru Yamaguchi et al. in 1979.
The Steglich esterification is a variation of an esterification with dicyclohexylcarbodiimide as a coupling reagent and 4-dimethylaminopyridine as a catalyst. The reaction was first described by Wolfgang Steglich in 1978. It is an adaptation of an older method for the formation of amides by means of DCC (dicyclohexylcarbodiimide) and 1-hydroxybenzotriazole (HOBT).

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field.

In organic chemistry, carbonyl reduction is the organic reduction of any carbonyl group by a reducing agent.
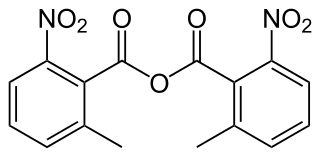
2-Methyl-6-nitrobenzoic anhydride is an organic acid anhydride also known as the Shiina reagent, having a structure wherein carboxylic acids undergo intermolecular dehydration condensation. It was developed in 2002 by Prof. Isamu Shiina. The compound is often abbreviated MNBA.
Shiina macrolactonization is an organic chemical reaction that synthesizes cyclic compounds by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic cyclization method using Lewis acid catalyst, and, in 2002, a basic cyclization using nucleophilic catalyst.
Shiina esterification is an organic chemical reaction that synthesizes carboxylic esters from nearly equal amounts of carboxylic acids and alcohols by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic coupling method using Lewis acid, and, in 2002, a basic esterification using nucleophilic catalyst.