
Mange is a type of skin disease caused by parasitic mites. Because various species of mites also infect plants, birds and reptiles, the term "mange", or colloquially "the mange", suggesting poor condition of the skin and fur due to the infection, is sometimes reserved for pathological mite-infestation of nonhuman mammals. Thus, mange includes mite-associated skin disease in domestic mammals, in livestock, and in wild mammals (for example, foxes, coyotes, cougars and wombats. Severe mange caused by mites has been observed in wild bears. Since mites belong to the arachnid subclass Acari, another term for mite infestation is acariasis.

Streptomyces is the largest genus of Actinomycetota, and the type genus of the family Streptomycetaceae. Over 700 species of Streptomyces bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have very large genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin. Different strains of the same species may colonize very diverse environments.

Ivermectin is an antiparasitic drug. After its discovery in 1975, its first uses were in veterinary medicine to prevent and treat heartworm and acariasis. Approved for human use in 1987, it is used to treat infestations including head lice, scabies, river blindness (onchocerciasis), strongyloidiasis, trichuriasis, ascariasis and lymphatic filariasis. It works through many mechanisms to kill the targeted parasites, and can be taken by mouth, or applied to the skin for external infestations. It belongs to the avermectin family of medications.
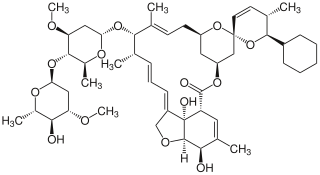
Doramectin, sold under the brand name Dectomax among others, is a veterinary medication approved by the US Food and Drug Administration (FDA) for the treatment of parasites such as gastrointestinal roundworms, lungworms, eyeworms, grubs, sucking lice, and mange mites in cattle. It is available as a generic medication. It is available as a combination with levamisole under the brand name Valcor.

Selamectin, sold under the brand name Revolution, among others, is a topical parasiticide and anthelminthic used on dogs and cats. It treats and prevents infections of heartworms, fleas, ear mites, sarcoptic mange (scabies), and certain types of ticks in dogs, and prevents heartworms, fleas, ear mites, hookworms, and roundworms in cats. It is structurally related to ivermectin and milbemycin. Selamectin is not approved for human use.

Moxidectin is an anthelmintic drug used in animals to prevent or control parasitic worms (helminths), such as heartworm and intestinal worms, in dogs, cats, horses, cattle and sheep. Moxidectin kills some of the most common internal and external parasites by selectively binding to a parasite's glutamate-gated chloride ion channels. These channels are vital to the function of invertebrate nerve and muscle cells; when moxidectin binds to the channels, it disrupts neurotransmission, resulting in paralysis and death of the parasite.

The avermectins are a series of drugs and pesticides used to treat parasitic worm infestations and to reduce insect pests. They are a group of 16-membered macrocyclic lactone derivatives with potent anthelmintic and insecticidal properties. These naturally occurring compounds are generated as fermentation products by Streptomyces avermitilis, a soil actinomycete. Eight different avermectins were isolated in four pairs of homologue compounds, with a major (a-component) and minor (b-component) component usually in ratios of 80:20 to 90:10. Avermectin B1, a mixture of B1a and B1b, is the drug and pesticide abamectin. Other anthelmintics derived from the avermectins include ivermectin, selamectin, doramectin, eprinomectin.

Spinosad is an insecticide based on chemical compounds found in the bacterial species Saccharopolyspora spinosa. The genus Saccharopolyspora was discovered in 1985 in isolates from crushed sugarcane. The bacteria produce yellowish-pink aerial hyphae, with bead-like chains of spores enclosed in a characteristic hairy sheath. This genus is defined as aerobic, Gram-positive, nonacid-fast actinomycetes with fragmenting substrate mycelium. S. spinosa was isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. Spinosad is a mixture of chemical compounds in the spinosyn family that has a generalized structure consisting of a unique tetracyclic ring system attached to an amino sugar (D-forosamine) and a neutral sugar (tri-Ο-methyl-L-rhamnose). Spinosad is relatively nonpolar and not easily dissolved in water.

Thelaziasis is the term for infestation with parasitic nematodes of the genus Thelazia. The adults of all Thelazia species discovered so far inhabit the eyes and associated tissues of various mammal and bird hosts, including humans. Thelazia nematodes are often referred to as "eyeworms".
The milbemycins are a group of macrolides chemically related to the avermectins and were first isolated in 1972 from Streptomyces hygroscopicus. They are used in veterinary medicine as antiparasitic agents against worms, ticks and fleas.

Milbemycin oxime, sold under the brand name Interceptor among others, is a veterinary medication from the group of milbemycins, used as a broad spectrum antiparasitic. It is active against worms (anthelmintic) and mites (miticide).

Anthelmintics or antihelminthics are a group of antiparasitic drugs that expel parasitic worms (helminths) and other internal parasites from the body by either stunning or killing them and without causing significant damage to the host. They may also be called vermifuges or vermicides. Anthelmintics are used to treat people who are infected by helminths, a condition called helminthiasis. These drugs are also used to treat infected animals.
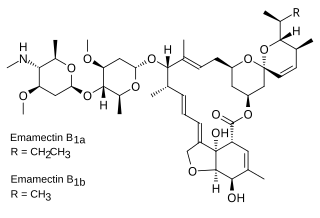
Emamectin is the 4″-deoxy-4″-methylamino derivative of abamectin, a 16-membered macrocyclic lactone produced by the fermentation of the soil actinomycete Streptomyces avermitilis. It is generally prepared as the salt with benzoic acid, emamectin benzoate, which is a white or faintly yellow powder. Emamectin is widely used in the US and Canada as an insecticide because of its chloride channel activation properties.

Oxfendazole is a broad spectrum benzimidazole anthelmintic. Its main use is for protecting livestock against roundworm, strongyles and pinworms. Oxfendazole is the sulfoxide metabolite of fenbendazole.
Streptomyces avermitilis is a species of bacteria in the genus Streptomyces. This bacterium was discovered by Satoshi Ōmura in Shizuoka Prefecture, Japan.

Satoshi Ōmura is a Japanese biochemist. He is known for the discovery and development of hundreds of pharmaceuticals originally occurring in microorganisms. In 2015, he was awarded the Nobel Prize in Physiology or Medicine jointly with William C. Campbell for their role in the discovery of avermectins and ivermectin, the world's first endectocide and a safe and highly effective microfilaricide. It is believed that the large molecular size of ivermectin prevents it from crossing the blood/aqueous humour barrier, and renders the drug an important treatment of helminthically-derived blindness.

William Cecil Campbell is an Irish biologist and parasitologist with United States citizenship, known for his work in discovering a novel therapy against infections caused by roundworms, for which he was jointly awarded the 2015 Nobel Prize in Physiology or Medicine. He helped to discover a class of drugs called avermectins, whose derivatives have been shown to have "extraordinary efficacy" in treating River blindness and Lymphatic filariasis, among other parasitic diseases affecting animals and humans. Campbell worked at the Merck Institute for Therapeutic Research 1957–1990, and is currently a research fellow emeritus at Drew University.
Streptomyces microflavus is a bacterium species from the genus of Streptomyces which has been isolated from soil. Streptomyces microflavus produces nemadectin, fattiviracin A1, milbemycin and deoxyuridines. Streptomyces microflavus also produces the ionophore valinomycin. Streptomyces microflavus is also known to cause potato common scab disease in Korea.

Eprinomectin is an avermectin used as a veterinary topical endectocide. It is a mixture of two chemical compounds, eprinomectin B1a and B1b.

Carlos E. Lanusse is an Argentine scientist and a professor of Pharmacology. He is the Director of the Veterinary Research Center and the Science and Technology Center of the Argentina National Council of Research in Tandil.



















