
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The chemical energy released is available under the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a "cycle", it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production.
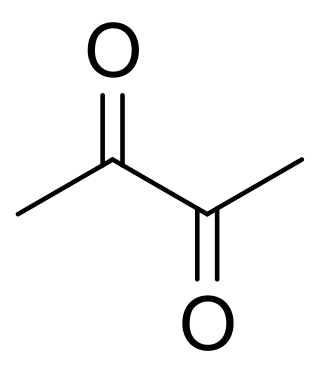
Diacetyl ( dy-yuh-SEE-tuhl; IUPAC systematic name: butanedione or butane-2,3-dione) is an organic compound with the chemical formula (CH3CO)2. It is a yellow liquid with an intensely buttery flavor. It is a vicinal diketone (two C=O groups, side-by-side). Diacetyl occurs naturally in alcoholic beverages and is added as a flavoring to some foods to impart its buttery flavor.

Pyruvate dehydrogenase complex (PDC) is a complex of three enzymes that converts pyruvate into acetyl-CoA by a process called pyruvate decarboxylation. Acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration, and this complex links the glycolysis metabolic pathway to the citric acid cycle. Pyruvate decarboxylation is also known as the "pyruvate dehydrogenase reaction" because it also involves the oxidation of pyruvate.

Malolactic conversion is a process in winemaking in which tart-tasting malic acid, naturally present in grape must, is converted to softer-tasting lactic acid. Malolactic fermentation is most often performed as a secondary fermentation shortly after the end of the primary fermentation, but can sometimes run concurrently with it. The process is standard for most red wine production and common for some white grape varieties such as Chardonnay, where it can impart a "buttery" flavor from diacetyl, a byproduct of the reaction.
Acetogenesis is a process through which acetate is produced by prokaryote microorganisms either by the reduction of CO2 or by the reduction of organic acids, rather than by the oxidative breakdown of carbohydrates or ethanol, as with acetic acid bacteria.
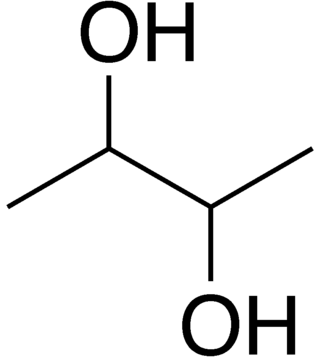
2,3-Butanediol fermentation is anaerobic fermentation of glucose with 2,3-butanediol as one of the end products. The overall stoichiometry of the reaction is

In biochemistry, mixed acid fermentation is the metabolic process by which a six-carbon sugar is converted into a complex and variable mixture of acids. It is an anaerobic (non-oxygen-requiring) fermentation reaction that is common in bacteria. It is characteristic for members of the Enterobacteriaceae, a large family of Gram-negative bacteria that includes E. coli.
Oxidative decarboxylation is a decarboxylation reaction caused by oxidation. Most are accompanied by α- Ketoglutarate α- Decarboxylation caused by dehydrogenation of hydroxyl carboxylic acids such as carbonyl carboxylic acid, malic acid, isocitric acid, etc.
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells. In addition to cytosolic fatty acid synthesis, there is also mitochondrial fatty acid synthesis (mtFASII), in which malonyl-CoA is formed from malonic acid with the help of malonyl-CoA synthetase (ACSF3), which then becomes the final product octanoyl-ACP (C8) via further intermediate steps.

In enzymology, a (R,R)-butanediol dehydrogenase (EC 1.1.1.4) is an enzyme that catalyzes the chemical reaction
In enzymology, a (S,S)-butanediol dehydrogenase (EC 1.1.1.76) is an enzyme that catalyzes the chemical reaction
In enzymology, an acetoin racemase is an enzyme that catalyzes the chemical reaction

The IMViC tests are a group of individual tests used in microbiology lab testing to identify an organism in the coliform group. A coliform is a gram negative, aerobic, or facultative anaerobic rod, which produces gas from lactose within 48 hours. The presence of some coliforms indicate fecal contamination.
Voges–Proskauer or VP is a test used to detect acetoin in a bacterial broth culture. The test is performed by adding alpha-naphthol and potassium hydroxide to the Voges-Proskauer broth, which is a glucose-phosphate broth that has been inoculated with bacteria. A cherry red color indicates a positive result, while a yellow-brown color indicates a negative result.
Glucose phosphate broth is used to perform methyl red (MR) test and Voges–Proskauer test (VP).
Acetoin dehydrogenase (EC 2.3.1.190, acetoin dehydrogenase complex, acetoin dehydrogenase enzyme system, AoDH ES) is an enzyme with systematic name acetyl-CoA:acetoin O-acetyltransferase. This enzyme catalyses the following chemical reaction
Diacetyl reductase ((R)-acetoin forming) (EC 1.1.1.303, (R)-acetoin dehydrogenase) is an enzyme with systematic name (R)-acetoin:NAD+ oxidoreductase. This enzyme catalyses the following chemical reaction

Diacetyl reductase ((S)-acetoin forming) (EC 1.1.1.304, (S)-acetoin dehydrogenase) is an enzyme with systematic name (S)-acetoin:NAD+ oxidoreductase. This enzyme catalyses the following chemical reaction

Acetylpropionyl, also known as acetyl propionyl or 2,3-pentanedione, is an organic compound, specifically a diketone.











