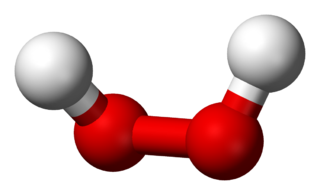
Hydrogen peroxide is a chemical compound with the formula H2O2. In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used both as a monopropellant and an oxidizer in rocketry.

In organic chemistry, a ketone is a functional group with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate.
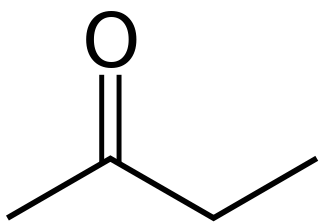
Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.
Unsymmetrical dimethylhydrazine (UDMH; 1,1-dimethylhydrazine, НДМГ or codenamed Geptil) is a chemical compound with the formula H2NN(CH3)2 that is used as a rocket propellant. It is a colorless liquid, with a sharp, fishy, ammonia-like smell typical for organic amines. Samples turn yellowish on exposure to air and absorb oxygen and carbon dioxide. It is miscible with water, ethanol, and kerosene. In concentration between 2.5% and 95% in air, its vapors are flammable. It is not sensitive to shock. Symmetrical dimethylhydrazine, 1,2-dimethylhydrazine is also known but is not as useful.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.

Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

Azo compounds are organic compounds bearing the functional group diazenyl.
The peroxide process is a method for the industrial production of hydrazine.

Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula [(CH3)2C(CN)]2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator.
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.
Hydrazides in organic chemistry are a class of organic compounds with the formula R−NR1−NR2R3 where R is acyl, sulfonyl, phosphoryl, phosphonyl and similar groups, R1, R2, R3 and R' are any groups. Unlike hydrazine and alkylhydrazines, hydrazides are nonbasic owing to the inductive influence of the acyl, sulfonyl, or phosphoryl substituent.

Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones. For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes.

Hydrazone iodination is an organic reaction in which a hydrazone is converted into a vinyl iodide by reaction of iodine and a non-nucleophilic base such as DBU. First published by Derek Barton in 1962 the reaction is sometimes referred to as the Barton reaction or, more descriptively, as the Barton vinyl iodine procedure.
Hydrazines (R2N−NR2) are a class of chemical compounds with two nitrogen atoms linked via a covalent bond and which carry from one up to four alkyl or aryl substituents. Hydrazines can be considered as derivatives of the inorganic hydrazine (H2N−NH2), in which one or more hydrogen atoms have been replaced by hydrocarbon groups.

Carbohydrazide is the chemical compound with the formula OC(N2H3)2. It is a white, water-soluble solid. It decomposes upon melting. A number of carbazides are known where one or more N-H groups are replaced by other substituents. They occur widely in the drugs, herbicides, plant growth regulators, and dyestuffs.

An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application, oxaziridines are intermediates in the industrial production of hydrazine. Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions. Oxaziridines also serve as precursors to amides and participate in [3+2] cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Chiral oxaziridine derivatives effect asymmetric oxygen transfer to prochiral enolates as well as other substrates. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center.

Acetone imine, or 2-propanimine is an organic compound and an imine with the chemical formula (CH3)2CNH. It is a volatile and flammable liquid at room temperature. It is the simplest ketimine. This compound is mainly of academic interest.






















