The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.

In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.

Rubidium is a chemical element; it has symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher than water. On Earth, natural rubidium comprises two isotopes: 72% is a stable isotope 85Rb, and 28% is slightly radioactive 87Rb, with a half-life of 48.8 billion years—more than three times as long as the estimated age of the universe.
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.

Ozonide is the polyatomic anion O−3. Cyclic organic compounds formed by the addition of ozone to an alkene are also called ozonides.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
The benzilic acid rearrangement is formally the 1,2-rearrangement of 1,2-diketones to form α-hydroxy–carboxylic acids using a base. This reaction receives its name from the reaction of benzil with potassium hydroxide to form benzilic acid. First performed by Justus von Liebig in 1838, it is the first reported example of a rearrangement reaction. It has become a classic reaction in organic synthesis and has been reviewed many times before. It can be viewed as an intramolecular redox reaction, as one carbon center is oxidized while the other is reduced.
Jack David Dunitz FRS was a British chemist and widely known chemical crystallographer. He was Professor of Chemical Crystallography at the ETH Zurich from 1957 until his official retirement in 1990. He held Visiting Professorships in the United States, Israel, Japan, Canada, Spain and the United Kingdom.
The Gattermann reaction (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as aluminium chloride (AlCl3). It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction.

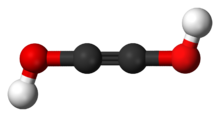
In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.
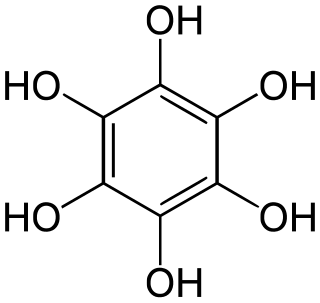
Benzenehexol, also called hexahydroxybenzene, is an organic compound with formula C
6H
6O
6 or C
6(OH)
6. It is a six-fold phenol of benzene. The product is also called hexaphenol, but this name has been used also for other substances.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
Mercury(II) hydroxide or mercuric hydroxide is the metal hydroxide with the chemical formula Hg(OH)2. The compound has not been isolated in pure form, although it has been the subject of several studies. Attempts to isolate Hg(OH)2 yield yellow solid HgO.
Selinenes are a group of closely related isomeric chemical compounds which are classified as sesquiterpenes. The selinenes all have the molecular formula C15H24 and they have been isolated from a variety of plant sources. α-Selinene and β-selinene are the most common and are two of the principal components of the oil from celery seeds. γ-Selinene and δ-selinene are less common.

2,3,4-Pentanetrione (or IUPAC name pentane-2,3,4-trione, triketopentane or dimethyl triketone) is the simplest linear triketone, a ketone with three C=O groups. It is an organic molecule with formula CH3COCOCOCH3.
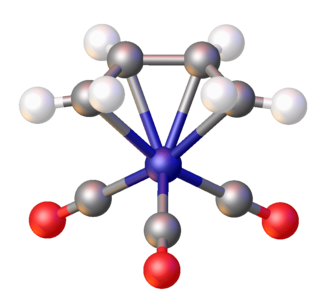
(Butadiene)iron tricarbonyl is an organoiron compound with the formula (C4H6)Fe(CO)3. It is a well-studied metal complex of butadiene. An orange-colored viscous liquid that freezes just below room temperature, the compound adopts a piano stool structure.
Rubidium permanganate is the permanganate salt of rubidium, with the chemical formula RbMnO
4.

Caesium permanganate is the permanganate salt of caesium, with the chemical formula CsMnO4.