
Acyl azides are carboxylic acid derivatives with the general formula RCON3. These compounds, which are a subclass of organic azides, are generally colorless. [1]

Acyl azides are carboxylic acid derivatives with the general formula RCON3. These compounds, which are a subclass of organic azides, are generally colorless. [1]
Typically acyl azides are generated under conditions where they rearrange to the isocyanate. [1]
Alkyl or aryl acyl chlorides react with sodium azide to give acyl azides. [2] [3]
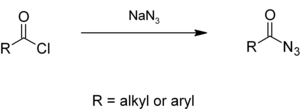
The second major route to azides is from the acyl hydrazides with nitrous acid. [1]
Acyl azides have also been synthesized from various carboxylic acids and sodium azide in presence of triphenylphosphine and trichloroacetonitrile catalysts in excellent yields at mild conditions. [4] Another route starts with aliphatic and aromatic aldehydes reacting with iodine azide which is formed from sodium azide and iodine monochloride in acetonitrile. [5]
On Curtius rearrangement, acyl azides yield isocyanates. [6] [7]
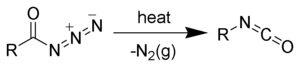
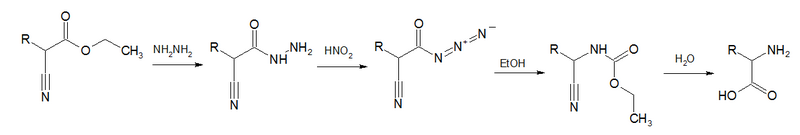
Acyl azides are also formed in Darapsky degradation, [8] [9] [10]
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N−. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds should not be confused with azo compounds or with diazonium compounds.
The Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one less carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.
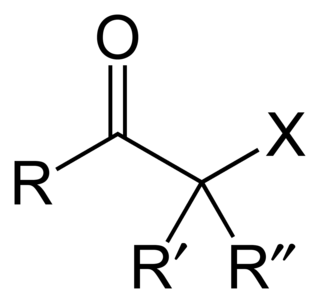
In organic chemistry, an α-halo ketone is a functional group consisting of a ketone group or more generally a carbonyl group with an α-halogen substituent. α-Halo ketones are alkylating agents. Prominent α-halo ketones include phenacyl bromide and chloroacetone.
The Hunsdiecker reaction is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide. It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material and a halogen atom is introduced its place. A catalytic approach has been developed.
The McFadyen–Stevens reaction is a chemical reaction best described as a base-catalyzed thermal decomposition of acylsulfonylhydrazides to aldehydes.
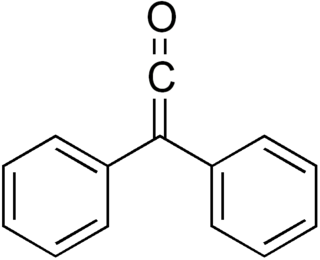
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most stable disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumulene. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.
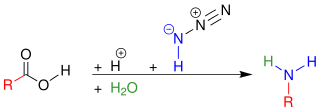
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. The intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products. The reaction is effective with carboxylic acids to give amines (above), and with ketones to give amides (below).
The Bergmann degradation is a series of chemical reactions designed to remove a single amino acid from the carboxylic acid (C-terminal) end of a peptide. First demonstrated by Max Bergmann in 1934, it is a rarely used method for sequencing peptides. The later developed Edman degradation is an improvement upon the Bergmann degradation, instead cleaving the N-terminal amino acid of peptides to produce a hydantoin containing the desired amino acid.
The Pfitzinger reaction is the chemical reaction of isatin with base and a carbonyl compound to yield substituted quinoline-4-carboxylic acids.
Nitrile anions is jargon from the organic product resulting from the deprotonation of alkylnitriles. The proton(s) α to the nitrile group are sufficiently acidic that they undergo deprotonation by strong bases, usually lithium-derived. The products are not anions but covalent organolithium complexes. Regardless, these organolithium compounds are reactive toward various electrophiles.
Fluorination by sulfur tetrafluoride produces organofluorine compounds from oxygen-containing organic functional groups using sulfur tetrafluoride. The reaction has broad scope, and SF4 is an inexpensive reagent. It is however hazardous gas whose handling requires specialized apparatus. Thus, for many laboratory scale fluorinations diethylaminosulfur trifluoride ("DAST") is used instead.
In organic chemistry, thiocarboxylic acids or carbothioic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a thione form and a thiol form. These are sometimes also referred to as "carbothioic O-acid" and "carbothioic S-acid" respectively. Of these the thiol form is most common.
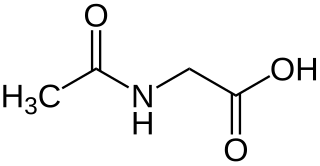
Aceturic acid (N-acetylglycine) is a derivative of the amino acid glycine. The conjugate base of this carboxylic acid is called aceturate, a term used for its esters and salts.
An organic azide is an organic compound that contains an azide functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" between an azide and an alkyne and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.
Lauroyl chloride is the organic compound with the formula CH3(CH2)10COCl. It is the acid chloride of lauric acid. Lauroyl chloride is a standard reagent for installing the lauroyl group. It is mainly produced as a precursor to dilauroyl peroxide, which is widely used in free-radical polymerizations.
{{cite book}}: CS1 maint: location missing publisher (link)