A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Glioblastoma, previously known as glioblastoma multiforme (GBM), is the most aggressive and most common type of cancer that originates in the brain, and has a very poor prognosis for survival. Initial signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness.

In medicine, proton therapy, or proton radiotherapy, is a type of particle therapy that uses a beam of protons to irradiate diseased tissue, most often to treat cancer. The chief advantage of proton therapy over other types of external beam radiotherapy is that the dose of protons is deposited over a narrow range of depth; hence in minimal entry, exit, or scattered radiation dose to healthy nearby tissues.

Lomustine is an alkylating nitrosourea compound used in chemotherapy. It is closely related to semustine and is in the same family as streptozotocin. It is a highly lipid-soluble drug, thus it crosses the blood–brain barrier. This property makes it ideal for treating brain tumors, which is its primary use, although it is also used to treat Hodgkin lymphoma as a second-line option. It has also been used in veterinary practice as a treatment for cancers in cats and dogs.

Cilengitide is a molecule designed and synthesized at the Technical University Munich in collaboration with Merck KGaA in Darmstadt. It is based on the cyclic peptide cyclo(-RGDfV-), which is selective for αv integrins, which are important in angiogenesis, and other aspects of tumor biology. Hence, it is under investigation for the treatment of glioblastoma, where it may act by inhibiting angiogenesis, and influencing tumor invasion and proliferation.

Paclitaxel trevatide is an experimental chemotherapy drug that is under development by Angiochem Inc, a Canadian biotech company. Phase II clinical trials have completed for several indications, and the company is preparing for phase III trials.
James L. Gulley is an American cancer researcher and the Director of the Medical Oncology Service at National Cancer Institute.
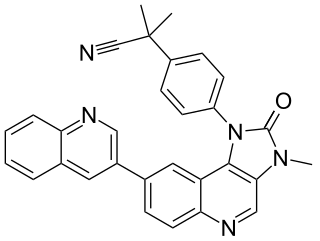
Dactolisib is an imidazoquinoline derivative acting as a PI3K inhibitor. It also inhibits mTOR. It is being investigated as a possible cancer treatment.
Peptide-based synthetic vaccines are subunit vaccines made from peptides. The peptides mimic the epitopes of the antigen that triggers direct or potent immune responses. Peptide vaccines can not only induce protection against infectious pathogens and non-infectious diseases but also be utilized as therapeutic cancer vaccines, where peptides from tumor-associated antigens are used to induce an effective anti-tumor T-cell response.
Gustav Gaudernack is a scientist working in the development of cancer vaccines and cancer immunotherapy. He has developed various strategies in immunological treatment of cancer. He is involved in several ongoing cellular and immuno-gene therapeutic clinical trials and his research group has put major efforts into the development of various T cell-based immunotherapeutic strategies.
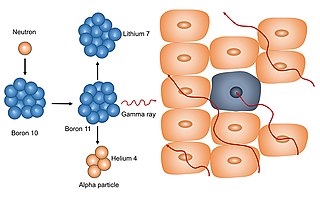
Neutron capture therapy (NCT) is a type of radiotherapy for treating locally invasive malignant tumors such as primary brain tumors, recurrent cancers of the head and neck region, and cutaneous and extracutaneous melanomas. It is a two-step process: first, the patient is injected with a tumor-localizing drug containing the stable isotope boron-10 (10B), which has a high propensity to capture low energy "thermal" neutrons. The neutron cross section of 10B is 1,000 times more than that of other elements, such as nitrogen, hydrogen, or oxygen, that occur in tissue. In the second step, the patient is radiated with epithermal neutrons, the sources of which in the past have been nuclear reactors and now are accelerators that produce higher energy epithermal neutrons. After losing energy as they penetrate tissue, the resultant low energy "thermal" neutrons are captured by the 10B atoms. The resulting decay reaction yields high-energy alpha particles that kill the cancer cells that have taken up enough 10B.

NeuVax is a peptide vaccine aimed at preventing or delaying the recurrence of breast cancer in cancer survivors who achieve remission after standard of care treatment. The product's developer is the US biotechnology company Galena Biopharma.
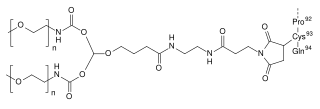
Pegdinetanib is an investigational anti-cancer drug that acts as a selective antagonist of vascular endothelial growth factor receptor 2 (VEGFR-2), hindering vascularization of tumors. It is a genetically engineered peptide derivative based on the monobody technology, and is being developed by Adnexus.

Sonidegib (INN), sold under the brand name Odomzo, is a medication used to treat cancer.
PROSTVAC is a cancer immunotherapy candidate in clinical development by Bavarian Nordic for the treatment of all prostate cancer although clinical trials are focusing on more advanced cases of metastatic castration-resistant prostate cancer (mCRPC). PROSTVAC is a vaccine designed to enable the immune system to recognize and attack prostate cancer cells by triggering a specific and targeted T cell immune response to cancer cells that express the tumor-associated antigen prostate-specific antigen (PSA).
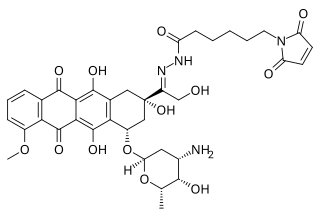
Aldoxorubicin (INNO-206) is a tumor-targeted doxorubicin conjugate in development by CytRx. Specifically, it is the (6-maleimidocaproyl) hydrazone of doxorubicin. Essentially, this chemical name describes doxorubicin attached to an acid-sensitive linker.
Eftilagimod alpha is a large-molecule cancer drug being developed by the clinical-stage biotechnology company Immutep. Efti is a soluble version of the immune checkpoint molecule LAG-3. It is an APC Activator used to increase an immune response to tumors, and is administered by subcutaneous injection. Efti has three intended clinical settings:
Varlilumab is a monoclonal antibody designed for immunotherapy for solid tumors and hematologic malignancies. It is an anti-CD27 antibody and helps activate T-cells.

Epitopoietic Research Corporation (ERC) is a Belgian Pharmaceutical company that is specialized in the development of ERC1671, a treatment for Glioblastoma multiforme, which is the most aggressive form of brain cancer. In 2019 ERC provided treatment under the US Federal Right-to-try law.

EpiVacCorona is a peptide-based vaccine against COVID-19 developed by the Russian VECTOR Center of Virology. The lack of protective effectiveness of EpiVacCorona, which is still in use in Russia, has been reported in scientific literature and in the media. The vaccine consists of three chemically synthesized peptides that are conjugated to a large carrier protein. This protein is a fusion product of a viral nucleocapsid protein and a bacterial MBP protein. A phase III clinical trial to show whether or not the vaccine can protect people against COVID-19 was launched in November 2020 with more than three thousand participants. The conclusions and results of the trial have not been made public.













