Related Research Articles

Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys. Metallurgy encompasses both the science and the technology of metals; that is, the way in which science is applied to the production of metals, and the engineering of metal components used in products for both consumers and manufacturers. Metallurgy is distinct from the craft of metalworking. Metalworking relies on metallurgy in a similar manner to how medicine relies on medical science for technical advancement. A specialist practitioner of metallurgy is known as a metallurgist.

Steel is an alloy made up of iron with typically a few tenths of a percent of carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant typically need an additional 11% chromium. Because of its high tensile strength and low cost, steel is used in buildings, infrastructure, tools, ships, trains, cars, machines, electrical appliances, weapons, and rockets. Iron is the base metal of steel. Depending on the temperature, it can take two crystalline forms : body-centred cubic and face-centred cubic. The interaction of the allotropes of iron with the alloying elements, primarily carbon, gives steel and cast iron their range of unique properties.

Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's resistance to corrosion results from the chromium, which forms a passive film that can protect the material and self-heal in the presence of oxygen.
Beryllium copper (BeCu), also known as copper beryllium (CuBe), beryllium bronze and spring copper, is a copper alloy with 0.5–3% beryllium, but can contain other elements as well. Beryllium copper combines high strength with non-magnetic and non-sparking qualities. It has excellent metalworking, forming and machining properties. It has many specialised applications in tools for hazardous environments, musical instruments, precision measurement devices, bullets, and aerospace. Beryllium alloys present a toxic inhalation hazard during manufacture.

Heat treating is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.

Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902); it exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures.

High-strength low-alloy steel (HSLA) is a type of alloy steel that provides better mechanical properties or greater resistance to corrosion than carbon steel. HSLA steels vary from other steels in that they are not made to meet a specific chemical composition but rather specific mechanical properties. They have a carbon content between 0.05 and 0.25% to retain formability and weldability. Other alloying elements include up to 2.0% manganese and small quantities of copper, nickel, niobium, nitrogen, vanadium, chromium, molybdenum, titanium, calcium, rare-earth elements, or zirconium. Copper, titanium, vanadium, and niobium are added for strengthening purposes. These elements are intended to alter the microstructure of carbon steels, which is usually a ferrite-pearlite aggregate, to produce a very fine dispersion of alloy carbides in an almost pure ferrite matrix. This eliminates the toughness-reducing effect of a pearlitic volume fraction yet maintains and increases the material's strength by refining the grain size, which in the case of ferrite increases yield strength by 50% for every halving of the mean grain diameter. Precipitation strengthening plays a minor role, too. Their yield strengths can be anywhere between 250–590 megapascals (36,000–86,000 psi). Because of their higher strength and toughness HSLA steels usually require 25 to 30% more power to form, as compared to carbon steels.

Stainless steels can be classified by their crystalline structure into five main types: austenitic, ferritic, martensitic, duplex, and precipitation hardened. Martensitic stainless steel is a specific type of stainless steel alloy that can be hardened and tempered through multiple ways of aging/heat treatment.

Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:

Maraging steels are steels that are known for possessing superior strength and toughness without losing ductility. Aging refers to the extended heat-treatment process. These steels are a special class of very-low-carbon ultra-high-strength steels that derive their strength not from carbon, but from precipitation of intermetallic compounds. The principal alloying element is 15 to 25 wt% nickel. Secondary alloying elements, which include cobalt, molybdenum and titanium, are added to produce intermetallic precipitates. Original development was carried out on 20 and 25 wt% Ni steels to which small additions of aluminium, titanium, and niobium were made; a rise in the price of cobalt in the late 1970s led to the development of cobalt-free maraging steels.

Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered at much higher temperatures.
Titanium alloys are alloys that contain a mixture of titanium and other chemical elements. Such alloys have very high tensile strength and toughness. They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures. However, the high cost of both raw materials and processing limit their use to military applications, aircraft, spacecraft, bicycles, medical devices, jewelry, highly stressed components such as connecting rods on expensive sports cars and some premium sports equipment and consumer electronics.
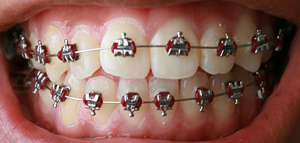
An archwire in orthodontics is a wire conforming to the alveolar or dental arch that can be used with dental braces as a source of force in correcting irregularities in the position of the teeth. An archwire can also be used to maintain existing dental positions; in this case it has a retentive purpose.
In metallurgy and materials science, annealing is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for an appropriate amount of time and then cooling.
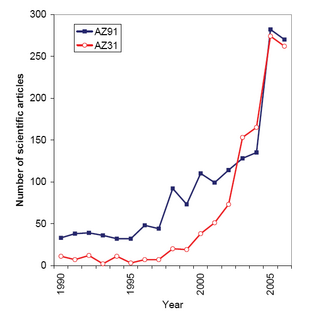
Magnesium alloys are mixtures of magnesium with other metals, often aluminium, zinc, manganese, silicon, copper, rare earths and zirconium. Magnesium alloys have a hexagonal lattice structure, which affects the fundamental properties of these alloys. Plastic deformation of the hexagonal lattice is more complicated than in cubic latticed metals like aluminium, copper and steel; therefore, magnesium alloys are typically used as cast alloys, but research of wrought alloys has been more extensive since 2003. Cast magnesium alloys are used for many components of modern automobiles and have been used in some high-performance vehicles; die-cast magnesium is also used for camera bodies and components in lenses.

The SAE steel grades system is a standard alloy numbering system for steel grades maintained by SAE International.
Eglin steel (ES-1) is a high-strength, high-performance, low-alloy, low-cost steel, developed for a new generation of bunker buster type bombs, e.g. the Massive Ordnance Penetrator and the improved version of the GBU-28 bomb known as EGBU-28. It was developed in collaboration between the US Air Force and the Ellwood National Forge Company.

Austempering is heat treatment that is applied to ferrous metals, most notably steel and ductile iron. In steel it produces a bainite microstructure whereas in cast irons it produces a structure of acicular ferrite and high carbon, stabilized austenite known as ausferrite. It is primarily used to improve mechanical properties or reduce / eliminate distortion. Austempering is defined by both the process and the resultant microstructure. Typical austempering process parameters applied to an unsuitable material will not result in the formation of bainite or ausferrite and thus the final product will not be called austempered. Both microstructures may also be produced via other methods. For example, they may be produced as-cast or air cooled with the proper alloy content. These materials are also not referred to as austempered.
Microalloyed steel is a type of alloy steel that contains small amounts of alloying elements, including niobium, vanadium, titanium, molybdenum, zirconium, boron, and rare-earth metals. They are used to refine the grain microstructure or facilitate precipitation hardening.
USAF-96 is a high-strength, high-performance, low-alloy, low-cost steel, developed for new generation of bunker buster type bombs, e.g. the Massive Ordnance Penetrator and the improved version of the GBU-28 bomb known as EGBU-28. It was developed by the US Air Force at the Eglin Air Force Munitions Directorate. It uses only materials domestic to the USA. In particular it requires no tungsten.
References
- ↑ Bhadeshia, H. K. D. H., Tempered Martensite, archived from the original on 2006-10-09, retrieved 2008-12-25.
- 1 2 3 4 5 6 AerMet 100 Alloy, 1995-09-01, retrieved 2008-08-29
- ↑ MIL-HDBK-5H, 1998-12-01, archived from the original on 2007-09-28, retrieved 2008-08-30
- 1 2 3 AerMet 310 Alloy, 2007-09-20, retrieved 2008-08-29
- 1 2 3 AerMet 340 Alloy, 2007-05-11, retrieved 2008-08-29