Cerebral edema is excess accumulation of fluid (edema) in the intracellular or extracellular spaces of the brain. This typically causes impaired nerve function, increased pressure within the skull, and can eventually lead to direct compression of brain tissue and blood vessels. Symptoms vary based on the location and extent of edema and generally include headaches, nausea, vomiting, seizures, drowsiness, visual disturbances, dizziness, and in severe cases, coma and death.

Glia, also called glial cells(gliocytes) or neuroglia, are non-neuronal cells in the central nervous system (brain and spinal cord) and the peripheral nervous system that do not produce electrical impulses. The neuroglia make up more than one half the volume of neural tissue in our body. They maintain homeostasis, form myelin in the peripheral nervous system, and provide support and protection for neurons. In the central nervous system, glial cells include oligodendrocytes, astrocytes, ependymal cells, and microglia, and in the peripheral nervous system they include Schwann cells and satellite cells.

Hyperammonemia is a metabolic disturbance characterised by an excess of ammonia in the blood. It is a dangerous condition that may lead to brain injury and death. It may be primary or secondary.

In haemodynamics, the body must respond to physical activities, external temperature, and other factors by homeostatically adjusting its blood flow to deliver nutrients such as oxygen and glucose to stressed tissues and allow them to function. Haemodynamic response (HR) allows the rapid delivery of blood to active neuronal tissues. The brain consumes large amounts of energy but does not have a reservoir of stored energy substrates. Since higher processes in the brain occur almost constantly, cerebral blood flow is essential for the maintenance of neurons, astrocytes, and other cells of the brain. This coupling between neuronal activity and blood flow is also referred to as neurovascular coupling.

Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries. The proportion of astrocytes in the brain is not well defined; depending on the counting technique used, studies have found that the astrocyte proportion varies by region and ranges from 20% to around 40% of all glia. Another study reports that astrocytes are the most numerous cell type in the brain. Astrocytes are the major source of cholesterol in the central nervous system. Apolipoprotein E transports cholesterol from astrocytes to neurons and other glial cells, regulating cell signaling in the brain. Astrocytes in humans are more than twenty times larger than in rodent brains, and make contact with more than ten times the number of synapses.

Hepatic encephalopathy (HE) is an altered level of consciousness as a result of liver failure. Its onset may be gradual or sudden. Other symptoms may include movement problems, changes in mood, or changes in personality. In the advanced stages it can result in a coma.

Astrogliosis is an abnormal increase in the number of astrocytes due to the destruction of nearby neurons from central nervous system (CNS) trauma, infection, ischemia, stroke, autoimmune responses or neurodegenerative disease. In healthy neural tissue, astrocytes play critical roles in energy provision, regulation of blood flow, homeostasis of extracellular fluid, homeostasis of ions and transmitters, regulation of synapse function and synaptic remodeling. Astrogliosis changes the molecular expression and morphology of astrocytes, in response to infection for example, in severe cases causing glial scar formation that may inhibit axon regeneration.

Glial fibrillary acidic protein (GFAP) is a protein that is encoded by the GFAP gene in humans. It is a type III intermediate filament (IF) protein that is expressed by numerous cell types of the central nervous system (CNS), including astrocytes and ependymal cells during development. GFAP has also been found to be expressed in glomeruli and peritubular fibroblasts taken from rat kidneys, Leydig cells of the testis in both hamsters and humans, human keratinocytes, human osteocytes and chondrocytes and stellate cells of the pancreas and liver in rats.
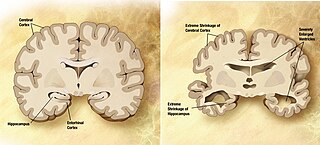
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.
Gliosis is a nonspecific reactive change of glial cells in response to damage to the central nervous system (CNS). In most cases, gliosis involves the proliferation or hypertrophy of several different types of glial cells, including astrocytes, microglia, and oligodendrocytes. In its most extreme form, the proliferation associated with gliosis leads to the formation of a glial scar.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The glia limitans, or the glial limiting membrane, is a thin barrier of astrocyte foot processes associated with the parenchymal basal lamina surrounding the brain and spinal cord. It is the outermost layer of neural tissue, and among its responsibilities is the prevention of the over-migration of neurons and neuroglia, the supporting cells of the nervous system, into the meninges. The glia limitans also plays an important role in regulating the movement of small molecules and cells into the brain tissue by working in concert with other components of the central nervous system (CNS) such as the blood–brain barrier (BBB).

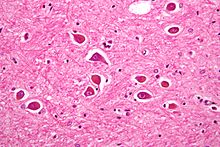
A gemistocyte is a swollen, reactive astrocyte.

Glutaminase is an amidohydrolase enzyme that generates glutamate from glutamine. Glutaminase has tissue-specific isoenzymes. Glutaminase has an important role in glial cells.

The subgranular zone (SGZ) is a brain region in the hippocampus where adult neurogenesis occurs. The other major site of adult neurogenesis is the subventricular zone (SVZ) in the brain.
Gliotransmitters are chemicals released from glial cells that facilitate neuronal communication between neurons and other glial cells. They are usually induced from Ca2+ signaling, although recent research has questioned the role of Ca2+ in gliotransmitters and may require a revision of the relevance of gliotransmitters in neuronal signalling in general.

A glial scar formation (gliosis) is a reactive cellular process involving astrogliosis that occurs after injury to the central nervous system. As with scarring in other organs and tissues, the glial scar is the body's mechanism to protect and begin the healing process in the nervous system.
In biochemistry, the glutamate–glutamine cycle is a cyclic metabolic pathway which maintains an adequate supply of the neurotransmitter glutamate in the central nervous system. Neurons are unable to synthesize either the excitatory neurotransmitter glutamate, or the inhibitory GABA from glucose. Discoveries of glutamate and glutamine pools within intercellular compartments led to suggestions of the glutamate–glutamine cycle working between neurons and astrocytes. The glutamate/GABA–glutamine cycle is a metabolic pathway that describes the release of either glutamate or GABA from neurons which is then taken up into astrocytes. In return, astrocytes release glutamine to be taken up into neurons for use as a precursor to the synthesis of either glutamate or GABA.
Potassium spatial buffering is a mechanism for the regulation of extracellular potassium concentration by astrocytes. Other mechanisms for astrocytic potassium clearance are carrier-operated or channel-operated potassium chloride uptake. The repolarization of neurons tends to raise potassium concentration in the extracellular fluid. If a significant rise occurs, it will interfere with neuronal signaling by depolarizing neurons. Astrocytes have large numbers of potassium ion channels facilitating the removal of potassium ions from the extracellular fluid. They are taken up at one region of the astrocyte and then distributed throughout the cytoplasm of the cell, and further to its neighbors via gap junctions. This keeps extracellular potassium at levels that prevent interference with the normal propagation of an action potential.

Alexei Verkhratsky, sometimes spelled Alexej, is a professor of neurophysiology at the University of Manchester best known for his research on the physiology and pathophysiology of neuroglia, calcium signalling, and brain ageing. He is an elected member and vice-president of Academia Europaea, of the German National Academy of Sciences Leopoldina, of the Real Academia Nacional de Farmacia (Spain), of the Slovenian Academy of Sciences and Arts, of Polish Academy of Sciences, and Dana Alliance for Brain Initiatives, among others. Since 2010, he is a Ikerbasque Research Professor and from 2012 he is deputy director of the Achucarro Basque Center for Neuroscience in Bilbao. He is a distinguished professor at Jinan University, China Medical University of Shenyang, and Chengdu University of Traditional Chinese Medicine and is an editor-in-chief of Cell Calcium, receiving editor for Cell Death and Disease, and Acta Physiologica and member of editorial board of many academic journals.