
Amyloid beta denotes peptides of 36–43 amino acids that are the main component of the amyloid plaques found in the brains of people with Alzheimer's disease. The peptides derive from the amyloid-beta precursor protein (APP), which is cleaved by beta secretase and gamma secretase to yield Aβ in a cholesterol-dependent process and substrate presentation. Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction akin to a prion infection. The oligomers are toxic to nerve cells. The other protein implicated in Alzheimer's disease, tau protein, also forms such prion-like misfolded oligomers, and there is some evidence that misfolded Aβ can induce tau to misfold.

Amyloid plaques are extracellular deposits of the amyloid beta (Aβ) protein mainly in the grey matter of the brain. Degenerative neuronal elements and an abundance of microglia and astrocytes can be associated with amyloid plaques. Some plaques occur in the brain as a result of aging, but large numbers of plaques and neurofibrillary tangles are characteristic features of Alzheimer's disease. The plaques are highly variable in shape and size; in tissue sections immunostained for Aβ, they comprise a log-normal size distribution curve, with an average plaque area of 400-450 square micrometers (µm²). The smallest plaques, which often consist of diffuse deposits of Aβ, are particularly numerous. Plaques form when Aβ misfolds and aggregates into oligomers and longer polymers, the latter of which are characteristic of amyloid.

Apolipoprotein E (Apo-E) is a protein involved in the metabolism of fats in the body of mammals. A subtype is implicated in the Alzheimer's disease and cardiovascular diseases. It is encoded in humans by the gene APOE.

A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, tauopathies, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.
A synthetic vaccine is a vaccine consisting mainly of synthetic peptides, carbohydrates, or antigens. They are usually considered to be safer than vaccines from bacterial cultures. Creating vaccines synthetically has the ability to increase the speed of production. This is especially important in the event of a pandemic.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.
The biochemistry of Alzheimer's disease, the most common cause of dementia, is not yet very well understood. Alzheimer's disease (AD) has been identified as a proteopathy: a protein misfolding disease due to the accumulation of abnormally folded amyloid beta (Aβ) protein in the brain. Amyloid beta is a short peptide that is an abnormal proteolytic byproduct of the transmembrane protein amyloid-beta precursor protein (APP), whose function is unclear but thought to be involved in neuronal development. The presenilins are components of proteolytic complex involved in APP processing and degradation.
Bapineuzumab is a humanized monoclonal antibody that acts on the nervous system and may have potential therapeutic value for the treatment of Alzheimer's disease and possibly glaucoma. However, in 2012 it failed to produce significant cognitive improvements in patients in two major trials, despite lowering key biomarkers of AD, amyloid brain plaque and hyperphosphorylated tau protein in CSF.
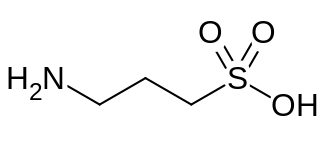
Homotaurine is a natural sulfonic acid found in seaweed. It is analogous to taurine, but with an extra carbon in its chain. It has GABAergic activity, apparently by mimicking GABA, which it resembles.

Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens, and is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems with language, disorientation, mood swings, loss of motivation, self-neglect, and behavioral issues. As a person's condition declines, they often withdraw from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the typical life expectancy following diagnosis is three to nine years.
Solanezumab is a monoclonal antibody being investigated by Eli Lilly as a neuroprotector for patients with Alzheimer's disease. The drug originally attracted extensive media coverage proclaiming it a breakthrough, but it has failed to show promise in Phase III trials.
Alzheimer's Disease Neuroimaging Initiative (ADNI) is a multisite study that aims to improve clinical trials for the prevention and treatment of Alzheimer's disease (AD). This cooperative study combines expertise and funding from the private and public sector to study subjects with AD, as well as those who may develop AD and controls with no signs of cognitive impairment. Researchers at 63 sites in the US and Canada track the progression of AD in the human brain with neuroimaging, biochemical, and genetic biological markers. This knowledge helps to find better clinical trials for the prevention and treatment of AD. ADNI has made a global impact, firstly by developing a set of standardized protocols to allow the comparison of results from multiple centers, and secondly by its data-sharing policy which makes available all at the data without embargo to qualified researchers worldwide. To date, over 1000 scientific publications have used ADNI data. A number of other initiatives related to AD and other diseases have been designed and implemented using ADNI as a model. ADNI has been running since 2004 and is currently funded until 2021.
Florbetaben, a fluorine-18 (18F)-labeled stilbene derivative, trade name NeuraCeq, is a diagnostic radiotracer developed for routine clinical application to visualize β-amyloid plaques in the brain. It is indicated for Positron Emission Tomography (PET) imaging of β-amyloid neuritic plaque density in the brains of adult patients with cognitive impairment who are being evaluated for Alzheimer's disease (AD) and other causes of cognitive impairment. β-amyloid is a key neuropathological hallmark of AD, so markers of β-amyloid plaque accumulation in the brain are useful in distinguishing AD from other causes of dementia. The tracer successfully completed a global multicenter phase 0–III development program and obtained approval in Europe, US and South Korea in 2014.
Lecanemab, sold under the brand name Leqembi, is a monoclonal antibody medication used for the treatment of Alzheimer's disease. Lecanemab is an amyloid beta-directed antibody. It is given via intravenous infusion. The most common side effects of lecanemab include headache, infusion-related reactions, and amyloid-related imaging abnormalities, a side effect known to occur with the class of antibodies targeting amyloid.
Donanemab is a biological drug in Phase III clinical trials to determine whether it slows the progression of early Alzheimer's disease. Donanemab has shown positive results in its first trials. Donanemab was developed by the Eli Lilly and Co. and is under clinical development as a possible treatment for Alzheimer's disease. There is currently no approved cure or disease-modifying treatment for Alzheimer's disease except for lecanemab.
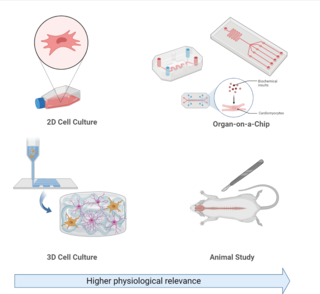
Experimental models of Alzheimer's disease are organism or cellular models used in research to investigate biological questions about Alzheimer's disease as well as develop and test novel therapeutic treatments. Alzheimer's disease is a progressive neurodegenerative disorder associated with aging, which occurs both sporadically or due to familial passed mutations in genes associated with Alzheimer's pathology. Common symptoms associated with Alzheimer's disease include: memory loss, confusion, and mood changes.

Simufilam (PTI-125) is an Investigational New Drug for the treatment of Alzheimer's disease. It is being developed by the American pharmaceutical firm Cassava Sciences. The drug is in phase III clinical trials as of October 2023. There are two phase III clinical studies: RETHINK-ALZ, a 52-week trial, is set to complete in 2024, and REFOCUS-ALZ, spanning 76 weeks, is projected to finish in 2025.
Alzheimer's disease (AD) in the Hispanic/Latino population is becoming a topic of interest in AD research as Hispanics and Latinos are disproportionately affected by Alzheimer's Disease and underrepresented in clinical research. AD is a neurodegenerative disease, characterized by the presence of amyloid-beta plaques and neurofibrillary tangles, that causes memory loss and cognitive decline in its patients. However, pathology and symptoms have been shown to manifest differently in Hispanic/Latinos, as different neuroinflammatory markers are expressed and cognitive decline is more pronounced. Additionally, there is a large genetic component of AD, with mutations in the amyloid precursor protein (APP), Apolipoprotein E APOE), presenilin 1 (PSEN1), bridging Integrator 1 (BIN1), SORL1, and Clusterin (CLU) genes increasing one's risk to develop the condition. However, research has shown these high-risk genes have a different effect on Hispanics and Latinos then they do in other racial and ethnic groups. Additionally, this population experiences higher rates of comorbidities, that increase their risk of developing AD. Hispanics and Latinos also face socioeconomic and cultural factors, such as low income and a language barrier, that affect their ability to engage in clinical trials and receive proper care.
Alzheimer's disease (AD) in African Americans is becoming a rising topic of interest in AD care, support, and scientific research, as African Americans are disproportionately affected by AD. Recent research on AD has shown that there are clear disparities in the disease among racial groups, with higher prevalence and incidence in African Americans than the overall average. Pathologies for Alzheimer’s also seem to manifest differently in African Americans, including with neuroinflammation markers, cognitive decline, and biomarkers. Although there are genetic risk factors for Alzheimer’s, these account for few cases in all racial groups.

Studies have shown that Alzheimer's disease (AD) patients are at an increased risk of morbidity and mortality from SARS-CoV-2, the virus that causes COVID-19. AD is the most common cause of dementia worldwide and is clinically defined by amyloid beta plaques, neurofibrillary tangles, and activation of the brain's immune system. While COVID-19 has been known to more severely impact elderly populations, AD patients have been shown to have a higher rate of SARS-CoV-2 infection compared to cognitively normal patients. The disproportionate risk of COVID-19 in AD patients is thought to arise from an interplay of biological and social factors between the two diseases. Many common biological pathways are shared between COVID-19 and AD, notably those involved in inflammation. Genetic factors that put individuals at risk for AD, such as the APOE4 genotype, are associated with worse outcomes during SARS-CoV-2 infection. Cognitive impairment in AD may prevent patients from following proper public health guidelines, such as masking and social distancing, increasing their risk of infection. Additionally, studies have shown cognitively normal COVID-19 patients are at an increased risk of AD diagnosis following recovery, suggesting that COVID-19 has the potential to cause AD.










