
The Controlled Substances Act (CSA) is the statute establishing federal U.S. drug policy under which the manufacture, importation, possession, use, and distribution of certain substances is regulated. It was passed by the 91st United States Congress as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 and signed into law by President Richard Nixon. The Act also served as the national implementing legislation for the Single Convention on Narcotic Drugs.

The Drug Enforcement Administration is a United States federal law enforcement agency under the U.S. Department of Justice tasked with combating illicit drug trafficking and distribution within the U.S. It is the lead agency for domestic enforcement of the Controlled Substances Act, sharing concurrent jurisdiction with the Federal Bureau of Investigation, the U.S. Immigration and Customs Enforcement, and U.S. Customs and Border Protection. However, the DEA has sole responsibility for coordinating and pursuing U.S. drug investigations both domestically and abroad.

Proposition 215, or the Compassionate Use Act of 1996, is a California law permitting the use of medical cannabis despite marijuana's lack of the normal Food and Drug Administration testing for safety and efficacy. It was enacted, on November 5, 1996, by means of the initiative process, and passed with 5,382,915 (55.6%) votes in favor and 4,301,960 (44.4%) against.
The Multidisciplinary Association for Psychedelic Studies (MAPS) is an American nonprofit organization working to raise awareness and understanding of psychedelic substances. MAPS was founded in 1986 by Rick Doblin and is now based in San Jose, California.
Jon B. Gettman is a marijuana rights activist, a leader of the Coalition for Rescheduling Cannabis, and a former head of the National Organization for the Reform of Marijuana Laws. He has a PhD in public policy and regional economic development from George Mason University and is a longtime contributor to High Times magazine. Gettman filed a petition in 1995 to remove cannabis from Schedule I of the Controlled Substances Act that was eventually denied. A second petition was filed in 2002, with the Coalition for Rescheduling Cannabis, that remains under review by the Department of Health and Human Services. Gettman frequently publishes on the marijuana industry and is an Associate Professor of Criminology and Criminal Justice at Shenandoah University in Virginia.
The Coalition for Rescheduling Cannabis is a U.S. organization founded c. 2002 to support removal of marijuana from Schedule I of the Controlled Substances Act. The group was organized immediately after the U.S. Court of Appeals denied the High Times/Jon Gettman petition to reschedule cannabis, ruling that the petitioners were not sufficiently injured to have standing to challenge the Drug Enforcement Administration's interpretation of the scientific record in federal court. On October 8, 2002, the Coalition filed a new petition to have cannabis rescheduled under federal law.

In the United States, the removal of cannabis from Schedule I of the Controlled Substances Act is a proposed legal and administrative change in cannabis-related law at the federal level. It has been proposed repeatedly since 1972. The category is the most tightly restricted category reserved for drugs that have "no currently accepted medical use.”
In United States v. Oakland Cannabis Buyers' Cooperative, 532 U.S. 483 (2001), the United States Supreme Court rejected the common-law medical necessity defense to crimes enacted under the federal Controlled Substances Act of 1970, regardless of their legal status under the laws of states such as California that recognize a medical use for marijuana. Oakland Cannabis Buyers' Cooperative was represented by Gerald Uelmen.
Medical necessity is a legal doctrine in the United States related to activities that may be justified as reasonable, necessary, and/or appropriate based on evidence-based clinical standards of care. In contrast, unnecessary health care lacks such justification.
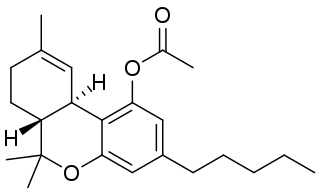
THC acetate ester is the acetate ester of THC. It is a metabolic pro-drug, with its subjective effects being felt around 30 minutes after ingestion.

In the United States, increased restrictions and labeling of cannabis as a poison began in many states from 1906 onward, and outright prohibitions began in the 1920s. By the mid-1930s cannabis was regulated as a drug in every state, including 35 states that adopted the Uniform State Narcotic Drug Act. The first national regulation was the Marihuana Tax Act of 1937.

The use, sale, and possession of cannabis over 0.3% THC in the United States, despite laws in many states permitting it under various circumstances, is illegal under federal law. As a Schedule I drug under the federal Controlled Substances Act (CSA) of 1970, cannabis over 0.3% THC is considered to have "no accepted medical use" and have a high potential for abuse and physical or psychological dependence. Cannabis use is illegal for any reason, with the exception of FDA-approved research programs. However, individual states have enacted legislation permitting exemptions for various uses, including medical, industrial, and recreational use.

Cannabis in Oregon is legal for both medical and recreational use. In recent decades, the U.S. state of Oregon has had a number of legislative, legal, and cultural events surrounding use of cannabis. Oregon was the first state to decriminalize the possession of small amounts of cannabis, and among the first to authorize its use for medical purposes. An attempt to recriminalize possession of small amounts of cannabis was turned down by Oregon voters in 1997.
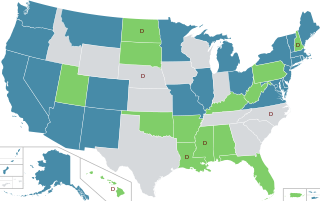
In the United States, the use of cannabis for medical purposes is legal in 38 states, four out of five permanently inhabited U.S. territories, and the District of Columbia, as of March 2023. Ten other states have more restrictive laws limiting THC content, for the purpose of allowing access to products that are rich in cannabidiol (CBD), a non-psychoactive component of cannabis. There is significant variation in medical cannabis laws from state to state, including how it is produced and distributed, how it can be consumed, and what medical conditions it can be used for.

The International Nonproprietary Name dronabinol, also known under the trade names Marinol, Syndros, Reduvo and Adversa, is a generic name for the molecule of delta-9-tetrahydrocannabinol in the pharmaceutical context. It has indications as an appetite stimulant, antiemetic, and sleep apnea reliever and is approved by the FDA as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting only.

Scott Feil is an American medical cannabis rights activist, complex aircraft pilot, and businessman. Most known for his involvement in the continuing court case involving Los Angeles Police Department illegal seizure of 209 pounds of medical marijuana, 21 pounds of hashish, 12 pounds of marijuana oil and amounts of U.S. legal tender amounting to $186,416.00 from his Los Angeles based United Medical Caregivers Clinic medical cannabis dispensary, UMCC LLC.

Conant v. Walters, 309 F.3d 629, is a legal case decided by the United States Court of Appeals for the Ninth Circuit, which affirmed the right of physicians to recommend medical marijuana. The Court of Appeals affirmed the earlier decision of the United States District Court for the Northern District of California, which was filed under the caption Conant v. McCaffrey. Though the case involved chronic patients with untreatable diseases, the decision does not name these conditions as a prerequisite, nor does it limit drugs which may or may not be illegal.
Patients Out of Time (POT) is an American medical cannabis nonprofit organization and patients rights group, established in 1995.
Medical cannabis research includes any medical research on using cannabis. Different countries conduct and respond to medical cannabis research in different ways.

Two main questions arise in the law surrounding driving after having ingested cannabis: (1) whether cannabis actually impairs driving ability, and (2) whether the common practice of testing for THC is a reliable means to measure impairment. On the first question, studies are mixed. Several recent, extensive studies–including one conducted by the National Highway Traffic Safety Administration and one conducted by the American Automobile Association (AAA)–show that drivers with detectable THC in their blood are no more likely to cause car crashes than drivers with no amount of THC in their blood. Others show that cannabis can impair certain abilities important to safe driving –but no studies have been able to show that this increases the actual risk of crashing, or that drivers with THC in their blood cause a disproportionate number of crashes. On the second question, the studies that have been conducted so far have consistently found that THC blood levels and degree of impairment are not closely related. No known relationship between blood levels of THC and increased relative crash risk, or THC blood levels and level of driving impairment, has been shown by single-crash or classic-control studies. Thus, even though it is possible that cannabis impairs driving ability to some extent, there are currently no reliable means to test or measure whether a driver was actually impaired.








