Related Research Articles

Adenosine triphosphate (ATP) is a nucleotide that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" of intracellular energy transfer.

Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine. It is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-.
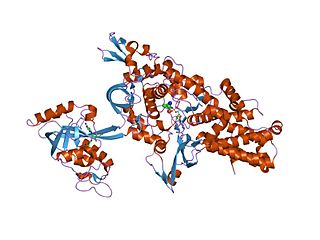
An aminoacyl-tRNA synthetase, also called tRNA-ligase, is an enzyme that attaches the appropriate amino acid onto its corresponding tRNA. It does so by catalyzing the transesterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. In humans, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid of the genetic code.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.
Activation, in chemistry and biology, is the process whereby something is prepared or excited for a subsequent reaction.

Aminoacyl-tRNA is tRNA to which its cognate amino acid is chemically bonded (charged). The aa-tRNA, along with particular elongation factors, deliver the amino acid to the ribosome for incorporation into the polypeptide chain that is being produced during translation.

Firefly luciferase is the light-emitting enzyme responsible for the bioluminescence of fireflies and click beetles. The enzyme catalyses the oxidation of firefly luciferin, requiring oxygen and ATP. Because of the requirement of ATP, firefly luciferases have been used extensively in biotechnology.

Aminoacyltransferases are acyltransferase enzymes which act upon an amino group. For instance, aminoacyl tRNA synthetases attach an aminoacid through esterification to the corresponding tRNA. The activation of amino acids it aminoacyl-tRNA synthetase requires hydrolysis of ATP to AMP plus PPi. The aminoacyl-tRNA molecule has close relationships with elongation facts like EF-Tu.
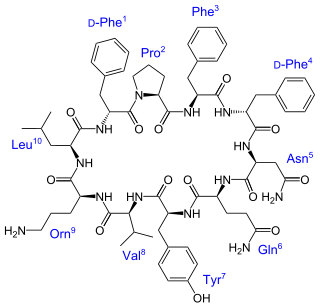
Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Brevibacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.
Protein metabolism denotes the various biochemical processes responsible for the synthesis of proteins and amino acids (anabolism), and the breakdown of proteins by catabolism.

The long chain fatty acyl-CoA ligase is an enzyme of the ligase family that activates the oxidation of complex fatty acids. Long chain fatty acyl-CoA synthetase catalyzes the formation of fatty acyl-CoA by a two-step process proceeding through an adenylated intermediate. The enzyme catalyzes the following reaction,
Aspartate—tRNAAsn ligase is an enzyme with systematic name L-aspartate:tRNAAsx ligase (AMP-forming). This enzyme catalyses the following chemical reaction
In enzymology, an isoleucine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a leucine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a lysine—tRNA ligase is an enzyme that catalyzes the chemical reaction

In enzymology, a phenylalanine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a threonine-tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a tryptophan-tRNA ligase is an enzyme that catalyzes the chemical reaction
Tyrosine—tRNA ligase, also known as tyrosyl-tRNA synthetase is an enzyme that is encoded by the gene YARS. Tyrosine—tRNA ligase catalyzes the chemical reaction
Cyclodipeptide synthases (CDPSs) are a newly defined family of peptide-bond forming enzymes that are responsible for the ribosome-independent biosynthesis of various cyclodipeptides, which are the precursors of many natural products with important biological activities. As a substrate for this synthesis, CDPSs use two amino acids activated as aminoacyl-tRNAs (aa-tRNAs), therefore diverting them from the ribosomal machinery. The first member of this family was identified in 2002 during the characterization of the albonoursin biosynthetic pathway in Streptomyces noursei. CDPSs are present in bacteria, fungi, and animal cells.
References
- 1 2 Pang YL, Poruri K, Martinis SA (2014). "tRNA synthetase: tRNA aminoacylation and beyond". WIREs RNA. 5 (4): 461–480. doi:10.1002/wrna.1224. PMC 4062602 . PMID 24706556.
- ↑ Alexander RW (2021-01-01). "Translation | tRNA Synthetases". In Jez J (ed.). Encyclopedia of Biological Chemistry III (Third ed.). Oxford: Elsevier. pp. 509–517. doi:10.1016/b978-0-12-819460-7.00257-7. ISBN 978-0-12-822040-5. S2CID 242703921.
- ↑ Kottur J, Nair DT (July 2018). "Pyrophosphate hydrolysis is an intrinsic and critical step of the DNA synthesis reaction". Nucleic Acids Research. 46 (12): 5875–5885. doi:10.1093/nar/gky402. PMC 6159520 . PMID 29850882.
- ↑ Swanson R, Hoben P, Sumner-Smith M, Uemura H, Watson L, Söll D (December 1988). "Accuracy of in vivo aminoacylation requires proper balance of tRNA and aminoacyl-tRNA synthetase". Science. 242 (4885): 1548–1551. Bibcode:1988Sci...242.1548S. doi:10.1126/science.3144042. PMID 3144042.
- ↑ Martínez-Bachs, Berta; Rimola, Albert (2019). "Prebiotic Peptide Bond Formation Through Amino Acid Phosphorylation. Insights from Quantum Chemical Simulations". Life. Vol. 9, no. 3. p. 75. doi: 10.3390/life9030075 – via MDPI.
- 1 2 Garrett, Reginald H.; Grisham, Charles M. (2017). Biochemistry (6th ed.). USA: Cengage Learning. pp. 1094–1098. ISBN 9781305886049.
- ↑ Martinez-Rodriguez, Luis (2015-08-07). "Functional Class I and II Amino Acid-activating Enzymes Can Be Coded by Opposite Strands of the Same Gene". Journal of Biological Chemistry. 290 (32): 19710–19725. doi: 10.1074/jbc.M115.642876 . ISSN 0021-9258. PMC 4528134 . PMID 26088142.
- ↑ Rybak, Mariia Yu; Rayevsky, Alexey V.; Gudzera, Olga I.; Tukalo, Michael A. (2019). "Stereospecificity control inaminoacyl-tRNA-synthetases: new evidence of D-amino acids activation and editing". Nucleic Acids Research. 47 (18): 9777–9788. doi:10.1093/nar/gkz756. PMC 6765224 . PMID 31504788.
- ↑ Beuning PJ, Musier-Forsyth K (August 2000). "Hydrolytic editing by a class II aminoacyl-tRNA synthetase". Proceedings of the National Academy of Sciences of the United States of America. 97 (16): 8916–8920. Bibcode:2000PNAS...97.8916B. doi: 10.1073/pnas.97.16.8916 . PMC 16796 . PMID 10922054.
- ↑ Jakubowski H (2011-11-17). "Quality control in tRNA charging". WIREs RNA. 3 (3): 295–310. doi:10.1002/wrna.122. PMID 22095844. S2CID 13450726.
- ↑ Kresge N, Simoni RD, Hill RL (June 2009). "The mechanism of amino acid activation: the work of Mahlon Hoagland. 1956". The Journal of Biological Chemistry. 284 (25): e7–e8. doi: 10.1016/S0021-9258(18)66554-8 . PMC 2719372 . PMID 19785094.