
Dynamite is an explosive made of nitroglycerin, sorbents and stabilizers. It was invented by the Swedish chemist and engineer Alfred Nobel in Geesthacht, Northern Germany and patented in 1867. It rapidly gained wide-scale use as a more powerful alternative to black powder.

An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

Trinitrotoluene more commonly known as TNT, or more specifically 2,4,6-trinitrotoluene, is a chemical compound with the formula C6H2(NO2)3CH3. This yellow solid is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

Picric acid is an organic compound with the formula (O2N)3C6H2OH. Its IUPAC name is 2,4,6-trinitrophenol (TNP). The name "picric" comes from the Greek word πικρός (pikros), meaning "bitter", due to its bitter taste. It is one of the most acidic phenols. Like other strongly nitrated organic compounds, picric acid is an explosive, which is its primary use. It has also been used as medicine (antiseptic, burn treatments) and as a dye.

Ammonium nitrate is a chemical compound with the chemical formula NH4NO3. It is a white crystalline solid consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Global production was estimated at 21.6 million tonnes in 2017.

ANFO is a widely used bulk industrial explosive. Its name is commonly pronounced as "ANN-foe".
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pharmaceuticals, pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.

Amatol is a highly explosive material made from a mixture of TNT and ammonium nitrate. The British name originates from the words ammonium and toluene. Similar mixtures were known as Schneiderite in France. Amatol was used extensively during World War I and World War II, typically as an explosive in military weapons such as aircraft bombs, shells, depth charges, and naval mines. It was eventually replaced with alternative explosives such as Composition B, Torpex, and tritonal.

Flash powder is a pyrotechnic composition, a mixture of oxidizer and metallic fuel, which burns quickly and if confined produces a loud noise. It is widely used in theatrical pyrotechnics and fireworks and was once used for flashes in photography.

Tannerite is a brand of binary explosive targets used for firearms practice and sold in kit form. The targets comprise a combination of oxidizers and a fuel, primarily aluminium powder, that is supplied as two separate components that are mixed by the user. The combination is relatively stable when subjected to forces less severe than a high-velocity bullet impact. A hammer blow, the product being dropped, or impact from a low-velocity bullet or shotgun blast will not initiate a reaction. It is also designed to be non-flammable, although its explosion can ignite flammable material.
An Oxyliquit, also called liquid air explosive or liquid oxygen explosive, is an explosive material which is a mixture of liquid oxygen (LOX) with a suitable fuel, such as carbon, or an organic chemical, wood meal, or aluminium powder or sponge. It is a class of Sprengel explosives.
Astrolite is the trade name of a family of explosives, invented by chemist Gerald Hurst in the 1960s during his employment with the Atlas Powder Company. The Astrolite family consists of two compounds, Astrolite G and Astrolite A. Both are two-part liquid-state high explosive mixtures, composed of ammonium nitrate oxidizer and hydrazine rocket fuel. The explosives were extensively studied, manufactured, and used in many countries because of their advantages of high energy, excellent performance, and wide application. They still find some use in commercial and civil blasting applications, but have mostly been superseded by cheaper and safer compounds, largely due to the expense and exceptionally poisonous nature of the hydrazine component.

There have been many extremely large explosions, accidental and intentional, caused by modern high explosives, boiling liquid expanding vapour explosions (BLEVEs), older explosives such as gunpowder, volatile petroleum-based fuels such as gasoline, and other chemical reactions. This list contains the largest known examples, sorted by date. An unambiguous ranking in order of severity is not possible; a 1994 study by historian Jay White of 130 large explosions suggested that they need to be ranked by an overall effect of power, quantity, radius, loss of life and property destruction, but concluded that such rankings are difficult to assess.
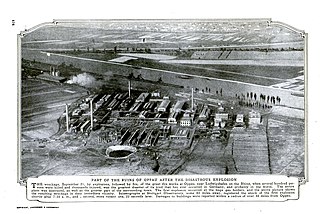
The Oppau explosion occurred on September 21, 1921, when approximately 4,500 tonnes of a mixture of ammonium sulfate and ammonium nitrate fertilizer stored in a tower silo exploded at a BASF plant in Oppau, now part of Ludwigshafen, Germany, killing 500–600 people and injuring about 2,000 more.
Minol is a military explosive developed by the British Admiralty early in the Second World War to augment supplies of trinitrotoluene (TNT) and RDX, which were then in short supply. The aluminium component in Minol significantly prolongs the explosive pulse, making it ideal for use in underwater naval weapons where munitions with a longer explosive pulse are more destructive than those with high brisance.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
Oxygen balance is an expression that is used to indicate the degree to which an explosive can be oxidized. If an explosive molecule contains just enough oxygen to form carbon dioxide from carbon, water from hydrogen atoms, all of its sulfur dioxide from sulfur, and all metal oxides from metals with no excess, the molecule is said to have a zero oxygen balance. The molecule is said to have a positive oxygen balance if it contains more oxygen than is needed and a negative oxygen balance if it contains less oxygen than is needed; the combustion will then be incomplete, and large amount of toxic gases like carbon monoxide will be present. The sensitivity, strength, and brisance of an explosive are all somewhat dependent upon oxygen balance and tend to approach their maxima as oxygen balance approaches zero.

The SC 250 was an air-dropped general purpose high-explosive bomb built by Germany during World War II and used extensively during that period. It could be carried by almost all German bomber aircraft, and was used to notable effect by the Junkers Ju 87 Stuka. The bomb's weight was about 250 kg, from which its designation was derived.

Tovex is a water-gel explosive composed of ammonium nitrate and methylammonium nitrate that has several advantages over traditional dynamite, including lower toxicity and safer manufacture, transport, and storage. It has thus almost entirely replaced dynamite. There are numerous versions ranging from shearing charges to aluminized common blasting agents. Tovex is used by 80% of international oil companies for seismic exploration.
Explosive materials are produced in numerous physical forms for their use in mining, engineering, or military applications. The different physical forms and fabrication methods are grouped together in several use forms of explosives.













