Related Research Articles
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.

In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a supersaturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.
The common-ion effect refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate. This behaviour is a consequence of Le Chatelier's principle for the equilibrium reaction of the ionic association/dissociation. The effect is commonly seen as an effect on the solubility of salts and other weak electrolytes. Adding an additional amount of one of the ions of the salt generally leads to increased precipitation of the salt, which reduces the concentration of both ions of the salt until the solubility equilibrium is reached. The effect is based on the fact that both the original salt and the other added chemical have one ion in common with each other.
A lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the labile macromolecules of the cells. Most lysis buffers contain buffering salts and ionic salts to regulate the pH and osmolarity of the lysate. Sometimes detergents are added to break up membrane structures. For lysis buffers targeted at protein extraction, protease inhibitors are often included, and in difficult cases may be almost required. Lysis buffers can be used on both animal and plant tissue cells.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants won't interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.
Salting out is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed.
A chaotropic agent is a molecule in water solution that can disrupt the hydrogen bonding network between water molecules. This has an effect on the stability of the native state of other molecules in the solution, mainly macromolecules by weakening the hydrophobic effect. For example, a chaotropic agent reduces the amount of order in the structure of a protein formed by water molecules, both in the bulk and the hydration shells around hydrophobic amino acids, and may cause its denaturation.
The first isolation of deoxyribonucleic acid (DNA) was done in 1869 by Friedrich Miescher. DNA extraction is the process of isolating DNA from the cells of an organism isolated from a sample, typically a biological sample such as blood, saliva, or tissue. It involves breaking open the cells, removing proteins and other contaminants, and purifying the DNA so that it is free of other cellular components. The purified DNA can then be used for downstream applications such as PCR, sequencing, or cloning. Currently, it is a routine procedure in molecular biology or forensic analyses.
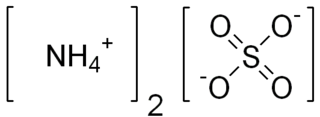
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

Ion chromatography is a form of chromatography that separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one unit away from the isoelectric point of a protein.
Co-solvents are defined as kosmotropic (order-making) if they contribute to the stability and structure of water-water interactions. In contrast, chaotropic (disorder-making) agents have the opposite effect, disrupting water structure, increasing the solubility of nonpolar solvent particles, and destabilizing solute aggregates. Kosmotropes cause water molecules to favorably interact, which in effect stabilizes intramolecular interactions in macromolecules such as proteins.
Ethanol precipitation is a method used to purify and/or concentrate RNA, DNA, and polysaccharides such as pectin and xyloglucan from aqueous solutions by adding ethanol as an antisolvent.

Veratridine is a steroidal alkaloid found in plants of the lily family, specifically the genera Veratrum and Schoenocaulon. Upon absorption through the skin or mucous membranes, it acts as a neurotoxin by binding to and preventing the inactivation of voltage-gated sodium ion channels in heart, nerve, and skeletal muscle cell membranes. Veratridine increases nerve excitability and intracellular Ca2+ concentrations.

The Hofmeister series or lyotropic series is a classification of ions in order of their lyotrophic properties, which is the ability to salt out or salt in proteins. The effects of these changes were first worked out by Franz Hofmeister, who studied the effects of cations and anions on the solubility of proteins.
Protein precipitation is widely used in downstream processing of biological products in order to concentrate proteins and purify them from various contaminants. For example, in the biotechnology industry protein precipitation is used to eliminate contaminants commonly contained in blood. The underlying mechanism of precipitation is to alter the solvation potential of the solvent, more specifically, by lowering the solubility of the solute by addition of a reagent.
Blood plasma fractionation are the general processes separating the various components of blood plasma, which in turn is a component of blood obtained through blood fractionation. Plasma-derived immunoglobulins are giving a new narrative to healthcare across a wide range of autoimmune inflammatory diseases. This widespread applicability is anticipated to leverage market prospects for plasma fractionation, pegged to witness a noteworthy 7% CAGR. COVID-19 pandemic is expected to generate growth opportunities for the plasma fractionation market.
Salting in refers to the effect where increasing the ionic strength of a solution increases the solubility of a solute, such as a protein. This effect tends to be observed at lower ionic strengths.
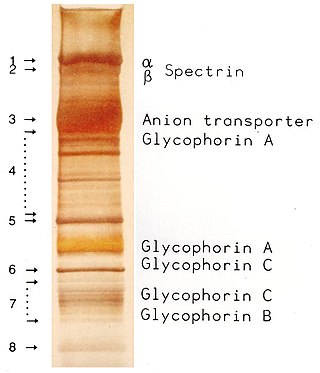
SDS-PAGE is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall.
References
- ↑ "Ammonium Sulfate". PubChem. National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved 2017-05-05.
- 1 2 3 Wingfield P (May 2001). "Protein precipitation using ammonium sulfate". Current Protocols in Protein Science. Appendix 3 (1): A.3F.1–A.3F.8. doi:10.1002/0471140864.psa03fs13. ISBN 0471140864. PMC 4817497 . PMID 18429073.
- 1 2 3 Duong-Ly KC, Gabelli SB (2014). "Salting out of proteins using ammonium sulfate precipitation". Laboratory Methods in Enzymology: Protein Part C. Vol. 541. pp. 85–94. doi:10.1016/B978-0-12-420119-4.00007-0. ISBN 9780124201194. PMID 24674064.
- 1 2 Burgess RR (2009). Protein precipitation techniques. Methods in Enzymology. Vol. 463. pp. 331–42. doi:10.1016/S0076-6879(09)63020-2. PMID 19892180.
- ↑ "Ammonium Sulfate Calculator". EnCor Biotechnology Inc. Retrieved 19 April 2013.
- ↑ Arslanian, Michael J.; Wakil, Salih J. (1975-01-01). "[7a] Fatty acid synthase from chicken liver". Lipids Part B. Methods in Enzymology. Vol. 35. pp. 59–65. doi:10.1016/0076-6879(75)35138-0. ISBN 9780121819354. ISSN 0076-6879. PMID 235706 . Retrieved 2020-10-31.
- ↑ Wang W, Liu QJ, Cui H (July 2007). "Rapid desalting and protein recovery with phenol after ammonium sulfate fractionation". Electrophoresis. 28 (14): 2358–60. doi:10.1002/elps.200600743. PMID 17577882. S2CID 33402573.