
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It was first documented in 1844 and came into medical use in 1867.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

Ceric ammonium nitrate (CAN) is the inorganic compound with the formula (NH4)2[Ce(NO3)6]. This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.
Potassium nitrite (distinct from potassium nitrate) is the inorganic compound with the chemical formula KNO2. It is an ionic salt of potassium ions K+ and nitrite ions NO2−, which forms a white or slightly yellow, hygroscopic crystalline powder that is soluble in water.

Copper chromite is an inorganic compound with the formula Cu2Cr2O5. It is a black solid that is used to catalyze reactions in organic synthesis.
Triethylborane (TEB), also called triethylboron, is an organoborane. It is a colorless pyrophoric liquid. Its chemical formula is (CH3CH2)3B or (C2H5)3B, abbreviated Et3B. It is soluble in organic solvents tetrahydrofuran and hexane.
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Diethylzinc (C2H5)2Zn, or DEZ, is a highly pyrophoric and reactive organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry. It is available commercially as a solution in hexanes, heptane, or toluene, or as a pure liquid.

Aluminium hydride is an inorganic compound with the formula AlH3. Alane and its derivatives are part of a family of common reducing reagents in organic synthesis based around group 13 hydrides. In solution—typically in etherial solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups, and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds. Given its density, and with hydrogen content on the order of 10% by weight, some forms of alane are, as of 2016, active candidates for storing hydrogen and so for power generation in fuel cell applications, including electric vehicles. As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis, e.g. of pharmaceuticals.

Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride.
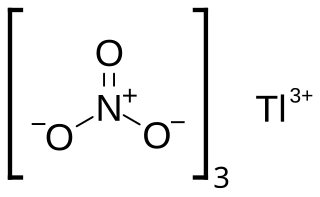
Thallium(III) nitrate, also known as thallic nitrate, is a thallium compound with chemical formula Tl(NO3)3. It is normally found as the trihydrate. It is a colorless and highly toxic salt. It is a strong oxidizing agent useful in organic synthesis. Among its many transformations, it oxidizes methoxyl phenols to quinone acetals, alkenes to acetals, and cyclic alkenes to ring-contracted aldehydes.
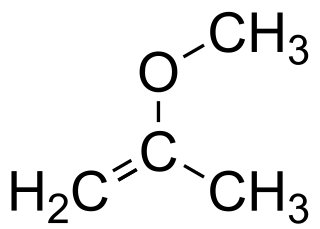
2-Methoxypropene is an ether with the chemical formula C4H8O. It is a reagent used in organic synthesis to introduce a protecting group for alcohols, and the conversion diols to the acetonide group.
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.

















