
Biogen Inc. is an American multinational biotechnology company based in Cambridge, Massachusetts, United States specializing in the discovery, development, and delivery of therapies for the treatment of neurological diseases to patients worldwide. Biogen operates in Argentina, Brazil, Canada, China, France, Germany, Hungary, India, Italy, Japan, Mexico, Netherlands, Poland, Sweden, and Switzerland.
Takeda Oncology is a biopharmaceutical company based in Cambridge, Massachusetts. It is a fully owned subsidiary of Takeda Pharmaceutical.

Asparaginase is an enzyme that is used as a medication and in food manufacturing. As a medication, L-asparaginase is used to treat acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL). It is given by injection into a vein, muscle, or under the skin. A pegylated version is also available. In food manufacturing it is used to decrease acrylamide.
Ocrelizumab, sold under the brand name Ocrevus, is a medication used for the treatment of multiple sclerosis (MS). It is a humanized anti-CD20 monoclonal antibody. It targets CD20 marker on B lymphocytes and is an immunosuppressive drug. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds.
Tocilizumab, sold under the brand name Actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, a severe form of arthritis in children, and COVID‑19. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). Interleukin 6 (IL-6) is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases, such as autoimmune diseases, multiple myeloma and prostate cancer. Tocilizumab was jointly developed by Osaka University and Chugai, and was licensed in 2003 by Hoffmann-La Roche.

The ALS Therapy Development Institute is a non-profit biotechnology research organization focused on finding treatments for amyotrophic lateral sclerosis (ALS). With a staff including more than 30 scientists, it operates a research and development program centered on ALS.

Pimavanserin, sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis and is also being studied for the treatment of Alzheimer's disease psychosis, schizophrenia, agitation, and major depressive disorder. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist.
Spectrum Pharmaceuticals is an American biopharmaceutical company located in Boston, MA. It develops and markets drugs for treatments in hematology and oncology.
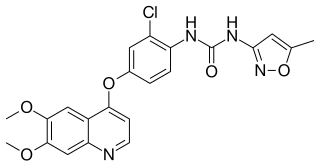
Tivozanib, sold under the brand name Fotivda, is a medication used for the treatment of advanced renal cell carcinoma. It is an oral VEGF receptor tyrosine kinase inhibitor.
Sarilumab, sold under the brand name Kevzara, is a human monoclonal antibody medication against the interleukin-6 receptor. Regeneron Pharmaceuticals and Sanofi developed the drug for the treatment of rheumatoid arthritis (RA), for which it received US FDA approval on 22 May 2017 and European Medicines Agency approval on 23 June 2017.

Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.
Alnylam Pharmaceuticals, Inc. is an American biopharmaceutical company focused on the discovery, development and commercialization of RNA interference (RNAi) therapeutics for genetically defined diseases. The company was founded in 2002 and is headquartered in Cambridge, Massachusetts. In 2016, Forbes included the company on its "100 Most Innovative Growth Companies" list.
Aducanumab, sold under the brand name Aduhelm, is a medication designed to treat Alzheimer's disease (AD). It is a monoclonal antibody that targets aggregated forms (plaque) of amyloid beta (Aβ) found in the brains of people with Alzheimer's disease to reduce its buildup. It was developed by Biogen and Eisai. Aducanumab is given via intravenous infusion.

Ionis Pharmaceuticals, Inc. is a biotechnology company based in Carlsbad, California, that specializes in discovering and developing RNA-targeted therapeutics. The company has three commercially approved medicines: Spinraza (Nusinersen), Tegsedi (Inotersen), and Waylivra (Volanesorsen) and has four drugs in pivotal studies: tominersen for Huntington's disease, tofersen for SOD1-ALS, AKCEA-APO(a)-LRx for cardiovascular disease, and AKCEA-TTR-LRx for all forms of TTR amyloidosis.
Genervon Biopharmaceuticals is a pharmaceutical company based in Pasadena, CA, focused on creating drugs for diseases of the central human nervous system. It is best known for its experimental drug, GM604, which seeks to treat Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease.

Erdafitinib, sold under the brand name Balversa, is an anti-cancer medication. It is a small molecule inhibitor of fibroblast growth factor receptor (FGFR) used for the treatment of cancer. FGFRs are a subset of tyrosine kinases which are unregulated in some tumors and influence tumor cell differentiation, proliferation, angiogenesis, and cell survival. Astex Pharmaceuticals discovered the drug and licensed it to Janssen Pharmaceuticals for further development.

COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID-19). From early 2020 through 2021, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing therapeutic candidates for COVID-19 disease in various stages of preclinical or clinical research, with 419 potential COVID-19 drugs in clinical trials, as of April 2021.
BeiGene, Ltd. is a global oncology company that specializes in the development of drugs for cancer treatment. Founded in 2010 by chief executive officer John V. Oyler and Xiaodong Wang, the multinational company headquartered in Cambridge, Massachusetts has offices in North America, Europe, South America, Asia and Australia. BeiGene has a large presence in Chinese market. BeiGene has developed several pharmaceuticals, including tislelizumab, a checkpoint inhibitor, and zanubrutinib, a Bruton's tyrosine kinase inhibitor.

CRISPR Therapeutics AG is a Swiss–American biotechnology company headquartered in Zug, Switzerland. It was one of the first companies formed to utilize the CRISPR gene editing platform to develop medicines for the treatment of various rare and common diseases. The company has approximately 500 employees and has offices in Zug, Switzerland, Boston, Massachusetts, San Francisco, California and London, United Kingdom. Its manufacturing facility in Framingham, Massachusetts won the Facilities of the Year Award (FOYA) award in 2022. The company’s lead program, exagamglogene autotemcel, or exa-cel, was granted regulatory approval by the US Food and Drug Administration (FDA) in December 2023.
Sodium phenylbutyrate/ursodoxicoltaurine, also known as sodium phenylbutyrate/taurursodiol and sold under the brand names Albrioza and Relyvrio, is a fixed-dose combination medication used for the treatment of amyotrophic lateral sclerosis (ALS). It contains sodium phenylbutyrate and ursodoxicoltaurine (taurursodiol).









