In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.
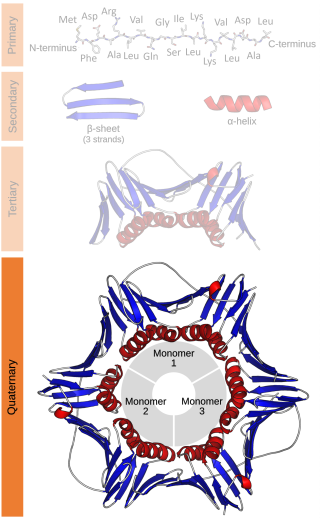
Protein quaternary structure is the fourth classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains. Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex. It includes organizations from simple dimers to large homooligomers and complexes with defined or variable numbers of subunits. In contrast to the first three levels of protein structure, not all proteins will have a quaternary structure since some proteins function as single units. Protein quaternary structure can also refer to biomolecular complexes of proteins with nucleic acids and other cofactors.

Size-exclusion chromatography, also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are commonly composed of dextran, agarose, or polyacrylamide polymers. The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.

A centrifuge is a device that uses centrifugal force to subject a specimen to a specified constant force, for example to separate various components of a fluid. This is achieved by spinning the fluid at high speed within a container, thereby separating fluids of different densities or liquids from solids. It works by causing denser substances and particles to move outward in the radial direction. At the same time, objects that are less dense are displaced and moved to the centre. In a laboratory centrifuge that uses sample tubes, the radial acceleration causes denser particles to settle to the bottom of the tube, while low-density substances rise to the top. A centrifuge can be a very effective filter that separates contaminants from the main body of fluid.

An ultracentrifuge is a centrifuge optimized for spinning a rotor at very high speeds, capable of generating acceleration as high as 1 000 000 g. There are two kinds of ultracentrifuges, the preparative and the analytical ultracentrifuge. Both classes of instruments find important uses in molecular biology, biochemistry, and polymer science.

A Svedberg unit or svedberg is a non-SI metric unit for sedimentation coefficients. The Svedberg unit offers a measure of a particle's size indirectly based on its sedimentation rate under acceleration. The svedberg is a measure of time, defined as exactly 10−13 seconds (100 fs).

Centrifugation is a mechanical process which involves the use of the centrifugal force to separate particles from a solution according to their size, shape, density, medium viscosity and rotor speed. The denser components of the mixture migrate away from the axis of the centrifuge, while the less dense components of the mixture migrate towards the axis. Chemists and biologists may increase the effective gravitational force of the test tube so that the precipitate (pellet) will travel quickly and fully to the bottom of the tube. The remaining liquid that lies above the precipitate is called a supernatant or supernate.

In biochemistry and cell biology, differential centrifugation is a common procedure used to separate organelles and other sub-cellular particles based on their sedimentation rate. Although often applied in biological analysis, differential centrifugation is a general technique also suitable for crude purification of non-living suspended particles. In a typical case where differential centrifugation is used to analyze cell-biological phenomena, a tissue sample is first lysed to break the cell membranes and release the organelles and cytosol. The lysate is then subjected to repeated centrifugations, where particles that sediment sufficiently quickly at a given centrifugal force for a given time form a compact "pellet" at the bottom of the centrifugation tube.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants will not interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.

Enzyme assays are laboratory methods for measuring enzymatic activity. They are vital for the study of enzyme kinetics and enzyme inhibition.
The sedimentation coefficient of a particle characterizes its sedimentation during centrifugation. It is defined as the ratio of a particle's sedimentation velocity to the applied acceleration causing the sedimentation.

A laboratory centrifuge is a piece of laboratory equipment, driven by a motor, which spins liquid samples at high speed. There are various types of centrifuges, depending on the size and the sample capacity.

Field-flow fractionation, abbreviated FFF, is a separation technique invented by J. Calvin Giddings. The technique is based on separation of colloidal or high molecular weight substances in liquid solutions, flowing through the separation platform, which does not have a stationary phase. It is similar to liquid chromatography, as it works on dilute solutions or suspensions of the solute, carried by a flowing eluent. Separation is achieved by applying a field or cross-flow, perpendicular to the direction of transport of the sample, which is pumped through a long and narrow laminar channel. The field exerts a force on the sample components, concentrating them towards one of the channel walls, which is called accumulation wall. The force interacts with a property of the sample, thereby the separation occurs, in other words, the components show differing "mobilities" under the force exerted by the crossing field. As an example, for the hydraulic, or cross-flow FFF method, the property driving separation is the translational diffusion coefficient or the hydrodynamic size. For a thermal field, it is the ratio of the thermal and the translational diffusion coefficient.
Counterflow centrifugal elutriation (CCE) is a liquid clarification technique. This method enables scientists to separate different cells with different sizes. Since cell size is correlated with cell cycle stages this method also allows the separation of cells at different stages of the cell cycle.

Affinity electrophoresis is a general name for many analytical methods used in biochemistry and biotechnology. Both qualitative and quantitative information may be obtained through affinity electrophoresis. Cross electrophoresis, the first affinity electrophoresis method, was created by Nakamura et al. Enzyme-substrate complexes have been detected using cross electrophoresis. The methods include the so-called electrophoretic mobility shift assay, charge shift electrophoresis and affinity capillary electrophoresis. The methods are based on changes in the electrophoretic pattern of molecules through biospecific interaction or complex formation. The interaction or binding of a molecule, charged or uncharged, will normally change the electrophoretic properties of a molecule. Membrane proteins may be identified by a shift in mobility induced by a charged detergent. Nucleic acids or nucleic acid fragments may be characterized by their affinity to other molecules. The methods have been used for estimation of binding constants, as for instance in lectin affinity electrophoresis or characterization of molecules with specific features like glycan content or ligand binding. For enzymes and other ligand-binding proteins, one-dimensional electrophoresis similar to counter electrophoresis or to "rocket immunoelectrophoresis", affinity electrophoresis may be used as an alternative quantification of the protein. Some of the methods are similar to affinity chromatography by use of immobilized ligands.

Bio-layer interferometry (BLI) is an optical biosensing technology that analyzes biomolecular interactions in real-time without the need for fluorescent labeling. Alongside Surface Plasmon Resonance, BLI is one of few widely available label-free biosensing technologies, a detection style that yields more information in less time than traditional processes. The technology relies on the phase shift-wavelength correlation created between interference patterns off of two unique surfaces on the tip of a biosensor. BLI has significant applications in quantifying binding strength, measuring protein interactions, and identifying properties of reaction kinetics, such as rate constants and reaction rates.
There are many methods to investigate protein–protein interactions which are the physical contacts of high specificity established between two or more protein molecules involving electrostatic forces and hydrophobic effects. Each of the approaches has its own strengths and weaknesses, especially with regard to the sensitivity and specificity of the method. A high sensitivity means that many of the interactions that occur are detected by the screen. A high specificity indicates that most of the interactions detected by the screen are occurring in reality.
The following outline is provided as an overview of and topical guide to biophysics:
Stephen E. Harding is a British biochemist specialising in biomolecular hydrodynamics. Harding is currently Professor of Applied Biochemistry at the University of Nottingham, has been the Director of the National Centre of Macromolecular Hydrodynamics since its foundation in 1987 and is a member of the Centre for the Study of the Viking Age.
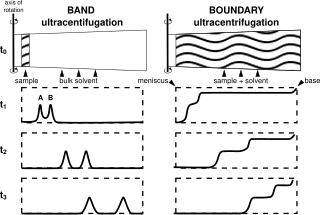
Analytical band centrifugation (ABC) (also known as analytical band ultracentrifugation, or band sedimentation-velocity), is a specialized ultracentrifugation procedure, where unlike the typical use of (boundary) sedimentation velocity analytical ultracentrifugation (SV-AUC) wherein a homogenous bulk solution is centrifuged, in ABC a thin (~15 µL, ~500 µm) sample is layered on top of a bulk solvent and then centrifuged. The method is distinguished from zone-sedimentation in that a stabilizing density gradient is self-generated during centrifugation, through the use of a higher density (than the sample) bulk "binary solvent", containing both a solvent (i.e. H2O), and a second component (small molecules, i.e. CsCl) that will sediment to form a stabilizing density gradient for the sample.












