Related Research Articles

The olfactory bulb is a neural structure of the vertebrate forebrain involved in olfaction, the sense of smell. It sends olfactory information to be further processed in the amygdala, the orbitofrontal cortex (OFC) and the hippocampus where it plays a role in emotion, memory and learning. The bulb is divided into two distinct structures: the main olfactory bulb and the accessory olfactory bulb. The main olfactory bulb connects to the amygdala via the piriform cortex of the primary olfactory cortex and directly projects from the main olfactory bulb to specific amygdala areas. The accessory olfactory bulb resides on the dorsal-posterior region of the main olfactory bulb and forms a parallel pathway. Destruction of the olfactory bulb results in ipsilateral anosmia, while irritative lesions of the uncus can result in olfactory and gustatory hallucinations.

The mushroom bodies or corpora pedunculata are a pair of structures in the brain of insects, other arthropods, and some annelids. They are also known to play a role in olfactory learning and memory. In most insects, the mushroom bodies and the lateral horn are the two higher brain regions that receive olfactory information from the antennal lobe via projection neurons. They were first identified and described by French biologist Félix Dujardin in 1850.

An olfactory receptor neuron (ORN), also called an olfactory sensory neuron (OSN), is a sensory neuron within the olfactory system.

The glomerulus is a spherical structure located in the olfactory bulb of the brain where synapses form between the terminals of the olfactory nerve and the dendrites of mitral, periglomerular and tufted cells. Each glomerulus is surrounded by a heterogeneous population of juxtaglomerular neurons and glial cells.
Olfactory receptors (ORs), also known as odorant receptors, are chemoreceptors expressed in the cell membranes of olfactory receptor neurons and are responsible for the detection of odorants which give rise to the sense of smell. Activated olfactory receptors trigger nerve impulses which transmit information about odor to the brain. These receptors are members of the class A rhodopsin-like family of G protein-coupled receptors (GPCRs). The olfactory receptors form a multigene family consisting of around 800 genes in humans and 1400 genes in mice.
The docking theory of olfaction proposes that the smell of an odorant molecule is due to a range of weak non-covalent interactions between the odorant [a ligand] and its protein odorant receptor, such as electrostatic and Van der Waals interactions as well as H-bonding, dipole attraction, pi-stacking, metal ion, Cation–pi interaction, and hydrophobic effects, in addition to odorant conformation. While this type of recognition has previously been termed the shape theory of olfaction, which primarily considers molecular shape and size, this latter model is oversimplified since two scent molecules may have similar shapes and sizes but different sets of weak intermolecular forces and therefore activate different combinations of odorant receptors. Earlier “lock and key” and "hand in glove" models of protein−ligand binding has been replaced by a more nuanced pictures which consider the distortion of flexible molecules so as to form the optimal interactions with binding partners as in molecular docking of non-olfactory G-protein coupled receptors.

Mitral cells are neurons that are part of the olfactory system. They are located in the olfactory bulb in the mammalian central nervous system. They receive information from the axons of olfactory receptor neurons, forming synapses in neuropils called glomeruli. Axons of the mitral cells transfer information to a number of areas in the brain, including the piriform cortex, entorhinal cortex, and amygdala. Mitral cells receive excitatory input from olfactory sensory neurons and external tufted cells on their primary dendrites, whereas inhibitory input arises either from granule cells onto their lateral dendrites and soma or from periglomerular cells onto their dendritic tuft. Mitral cells together with tufted cells form an obligatory relay for all olfactory information entering from the olfactory nerve. Mitral cell output is not a passive reflection of their input from the olfactory nerve. In mice, each mitral cell sends a single primary dendrite into a glomerulus receiving input from a population of olfactory sensory neurons expressing identical olfactory receptor proteins, yet the odor responsiveness of the 20-40 mitral cells connected to a single glomerulus is not identical to the tuning curve of the input cells, and also differs between sister mitral cells. Odorant response properties of individual neurons in an olfactory glomerular module. The exact type of processing that mitral cells perform with their inputs is still a matter of controversy. One prominent hypothesis is that mitral cells encode the strength of an olfactory input into their firing phases relative to the sniff cycle. A second hypothesis is that the olfactory bulb network acts as a dynamical system that decorrelates to differentiate between representations of highly similar odorants over time. Support for the second hypothesis comes primarily from research in zebrafish.
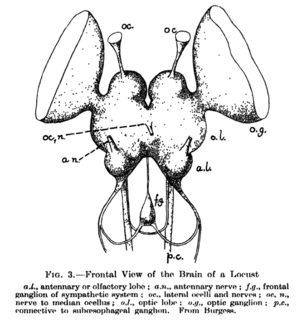
The supraesophageal ganglion is the first part of the arthropod and (especially) insect central nervous system. It receives and processes information from the first, second, and third metameres. The supraesophageal ganglion lies dorsal to the esophagus and consists of three parts, each a pair of ganglia that may be more or less pronounced, reduced, or fused depending on the genus:
A topographic map is the ordered projection of a sensory surface, like the retina or the skin, or an effector system, like the musculature, to one or more structures of the central nervous system. Topographic maps can be found in all sensory systems and in many motor systems.
Odorant-binding proteins (OBPs) are small soluble proteins secreted by auxiliary cells surrounding olfactory receptor neurons, including the nasal mucus of many vertebrate species and in the sensillar lymph of chemosensory sensilla of insects. OBPs are characterized by a specific protein domain that comprises six α-helices joined by three disulfide bonds. Although the function of the OBPs as a whole is not well established, it is believed that they act as odorant transporters, delivering the odorant molecules to olfactory receptors in the cell membrane of sensory neurons.
Dysosmia is a disorder described as any qualitative alteration or distortion of the perception of smell. Qualitative alterations differ from quantitative alterations, which include anosmia and hyposmia. Dysosmia can be classified as either parosmia or phantosmia. Parosmia is a distortion in the perception of an odorant. Odorants smell different from what one remembers. Phantosmia is the perception of an odor when no odorant is present. The cause of dysosmia still remains a theory. It is typically considered a neurological disorder and clinical associations with the disorder have been made. Most cases are described as idiopathic and the main antecedents related to parosmia are URTIs, head trauma, and nasal and paranasal sinus disease. Dysosmia tends to go away on its own but there are options for treatment for patients that want immediate relief.
Kenyon cells are the intrinsic neurons of the mushroom body, a neuropil found in the brains of most arthropods and some annelids. They were first described by F. C. Kenyon in 1896. The number of Kenyon cells in an organism varies greatly between species. For example, in the fruit fly, Drosophila melanogaster, there are about 2,500 Kenyon cells per mushroom body, while in cockroaches there are about 230,000.

The sense of smell, or olfaction, is the special sense through which smells are perceived. The sense of smell has many functions, including detecting hazards, and pheromones, and plays a role in taste.

Leslie Birgit Vosshall is an American neurobiologist and currently an HHMI Investigator and the Robin Chemers Neustein Professor of Neurogenetics and Behavior at The Rockefeller University. She is also the director of the Kavli Neural Systems Institute at The Rockefeller University. She is well known for her contributions to the field of olfaction, particularly for the discovery and subsequent characterization of the insect olfactory receptor family, and the genetic basis of chemosensory behavior in mosquitoes and humans.
Slit-Robo is the name of a cell signaling protein complex with many diverse functions including axon guidance and angiogenesis.
The lateral horn is one of the two areas of the insect brain where projection neurons of the antennal lobe send their axons. The other area is the mushroom body. Several morphological classes of neurons in the lateral horn receive olfactory information through the projection neurons.
Or83b, also known as Orco, is an odorant receptor and the corresponding gene that encodes it. The odorant receptor Or83b is not exclusively expressed in insects. Though its actual function is still a mystery, the broadly expressed Or83b has been conserved across highly divergent insect populations across 250 million years of evolution.

Insect olfaction refers to the function of chemical receptors that enable insects to detect and identify volatile compounds for foraging, predator avoidance, finding mating partners and locating oviposition habitats. Thus, it is the most important sensation for insects. Most important insect behaviors must be timed perfectly which is dependent on what they smell and when they smell it. For example, olfaction is essential for hunting in many species of wasps, including Polybia sericea.
Insect olfactory receptors are expressed in the cell membranes of the olfactory sensory neurons of insects. Similarly to mammalian olfactory receptors, in insects each olfactory sensory neuron expresses one type of OR, allowing the specific detection of a volatile chemical. Differently to mammalian ORs, insect ORs form a heteromer with a fixed monomer, Orco, and a variable OR monomer, which confers the odour specificity.

Reinhard F. Stocker is a Swiss biologist. He pioneered the analysis of the sense of smell and taste in higher animals, using the fly Drosophila melanogaster as a study case. He provided a detailed account of the anatomy and development of the olfactory system, in particular across metamorphosis, for which he received the Théodore-Ott-Prize of the Swiss Academy of Medical Sciences in 2007, and pioneered the use of larval Drosophila for the brain and behavioural sciences.
References
- 1 2 Scott, Kristin; Brady, Roscoe; Cravchik, Anibal; Morozov, Pavel; Rzhetsky, Andrey; Zuker, Charles; Axel, Richard (March 2001). "A Chemosensory Gene Family Encoding Candidate Gustatory and Olfactory Receptors in Drosophila". Cell. 104 (5): 661–673. doi:10.1016/S0092-8674(01)00263-X. PMID 11257221.
- 1 2 3 4 B. S. Hansson & S. Anton (2000). "Function and morphology of the antennal lobe: new developments". Annual Review of Entomology . 45: 203–231. doi:10.1146/annurev.ento.45.1.203. PMID 10761576.
- ↑ Leslie B. Vosshall, Allan M. Wong & Richard Axel (2000). "An olfactory sensory map in the fly brain". Cell . 102 (2): 147–159. doi:10.1016/S0092-8674(00)00021-0. PMID 10943836.
- ↑ Gregory S. X. E. Jefferis, Elizabeth C. Marin, Reinhard F. Stocker & Liqun Luo (2001). "Target neuron prespecification in the olfactory map of Drosophila" (PDF). Nature . 414 (6860): 204–208. Bibcode:2001Natur.414..204J. doi:10.1038/35102574. PMID 11719930.CS1 maint: multiple names: authors list (link)
- ↑ Watanabe, Hidehiro; Nishino, Hiroshi; Mizunami, Makoto; Yokohari, Fumio (5 May 2017). "Two Parallel Olfactory Pathways for Processing General Odors in a Cockroach". Frontiers in Neural Circuits. 11: 32. doi:10.3389/fncir.2017.00032. PMC 5418552 . PMID 28529476.
- ↑ Mark Stopfer, Vivek Jayaraman & Gilles Laurent (2003). "Intensity versus identity coding in an olfactory system". Neuron . 39 (6): 991–1004. doi:10.1016/j.neuron.2003.08.011. PMID 12971898.
- ↑ Gronenberg, W.; López-Riquelme, G.O. (February 2014). "Multisensory convergence in the mushroom bodies of ants and bees". Acta Biologica Hungarica. 55 (1–4): 31–37. doi:10.1556/ABiol.55.2004.1-4.5. PMID 15270216.
- ↑ López-Riquelme, G.O. (June 2014). "Odotopic afferent representation of the glomerular antennal lobe organization in the mushroom bodies of ants (Hymenoptera: Formicidae): Comparisons between two species". TIP Revista Especializada en Ciencias Químico-Biológicas. 17 (1): 15–31. doi:10.1016/S1405-888X(14)70317-1.
- ↑ Gilles Laurent (2002). "Olfactory network dynamics and the coding of multidimensional signals". Nature Reviews Neuroscience . 3 (11): 884–895. doi:10.1038/nrn964. PMID 12415296.
- ↑ Mark Stopfer & Gilles Laurent (1999). "Short-term memory in olfactory network dynamics". Nature . 402 (6762): 664–668. Bibcode:1999Natur.402..664S. doi:10.1038/45244. PMID 10604472.