
Acute disseminated encephalomyelitis (ADEM), or acute demyelinating encephalomyelitis, is a rare autoimmune disease marked by a sudden, widespread attack of inflammation in the brain and spinal cord. As well as causing the brain and spinal cord to become inflamed, ADEM also attacks the nerves of the central nervous system and damages their myelin insulation, which, as a result, destroys the white matter. The cause is often a trigger such as from viral infection or vaccinations.
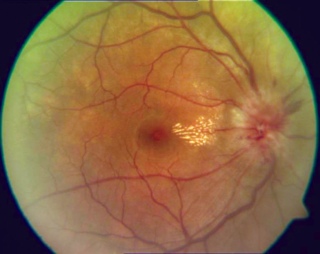
Optic neuritis describes any condition that causes inflammation of the optic nerve; it may be associated with demyelinating diseases, or infectious or inflammatory processes.

Transverse myelitis (TM) is a rare neurological condition wherein the spinal cord is inflamed. The adjective transverse implies that the spinal inflammation (myelitis) extends horizontally throughout the cross section of the spinal cord; the terms partial transverse myelitis and partial myelitis are sometimes used to specify inflammation that affects only part of the width of the spinal cord. TM is characterized by weakness and numbness of the limbs, deficits in sensation and motor skills, dysfunctional urethral and anal sphincter activities, and dysfunction of the autonomic nervous system that can lead to episodes of high blood pressure. Signs and symptoms vary according to the affected level of the spinal cord. The underlying cause of TM is unknown. The spinal cord inflammation seen in TM has been associated with various infections, immune system disorders, or damage to nerve fibers, by loss of myelin. As opposed to leukomyelitis which affects only the white matter, it affects the entire cross-section of the spinal cord. Decreased electrical conductivity in the nervous system can result.
Multiplesclerosis (MS) is the most common demyelinating disease, in which the insulating covers of nerve cells in the brain and spinal cord are damaged. This damage disrupts the ability of parts of the nervous system to transmit signals, resulting in a range of signs and symptoms, including physical, mental, and sometimes psychiatric problems. Specific symptoms can include double vision, visual loss, muscle weakness, and trouble with sensation or coordination. MS takes several forms, with new symptoms either occurring in isolated attacks or building up over time. In the relapsing forms of MS, between attacks, symptoms may disappear completely, although some permanent neurological problems often remain, especially as the disease advances.
Neuromyelitis optica spectrum disorders (NMOSD), including neuromyelitis optica (NMO), are autoimmune diseases characterized by acute inflammation of the optic nerve and the spinal cord (myelitis). Episodes of ON and myelitis can be simultaneous or successive. A relapsing disease course is common, especially in untreated patients. In more than 80% of cases, NMO is caused by immunoglobulin G autoantibodies to aquaporin 4 (anti-AQP4), the most abundant water channel protein in the central nervous system. A subset of anti-AQP4-negative cases is associated with antibodies against myelin oligodendrocyte glycoprotein (anti-MOG). Rarely, NMO may occur in the context of other autoimmune diseases or infectious diseases. In some cases, the etiology remains unknown.

Myelin oligodendrocyte glycoprotein (MOG) is a glycoprotein believed to be important in the myelination of nerves in the central nervous system (CNS). In humans this protein is encoded by the MOG gene. It is speculated to serve as a necessary "adhesion molecule" to provide structural integrity to the myelin sheath and is known to develop late on the oligodendrocyte.

Multiple sclerosis is an inflammatory demyelinating disease of the CNS in which activated immune cells invade the central nervous system and cause inflammation, neurodegeneration, and tissue damage. The underlying cause is currently unknown. Current research in neuropathology, neuroimmunology, neurobiology, and neuroimaging, together with clinical neurology, provide support for the notion that MS is not a single disease but rather a spectrum.
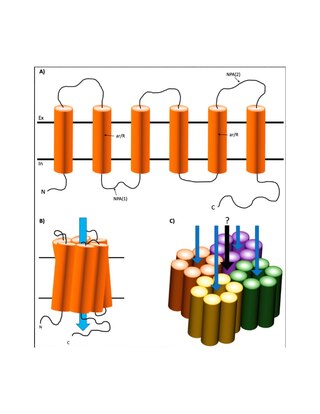
Aquaporin-4, also known as AQP-4, is a water channel protein encoded by the AQP4 gene in humans. AQP-4 belongs to the aquaporin family of integral membrane proteins that conduct water through the cell membrane. A limited number of aquaporins are found within the central nervous system (CNS): AQP1, 3, 4, 5, 8, 9, and 11, but more exclusive representation of AQP1, 4, and 9 are found in the brain and spinal cord. AQP4 shows the largest presence in the cerebellum and spinal cord grey matter. In the CNS, AQP4 is the most prevalent aquaporin channel, specifically located at the perimicrovessel astrocyte foot processes, glia limitans, and ependyma. In addition, this channel is commonly found facilitating water movement near cerebrospinal fluid and vasculature.
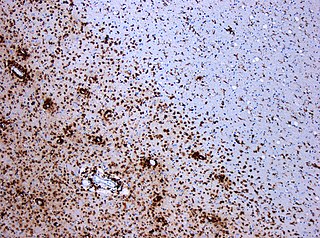
Multiple sclerosis and other demyelinating diseases of the central nervous system (CNS) produce lesions and glial scars or scleroses. They present different shapes and histological findings according to the underlying condition that produces them.
Inflammatory demyelinating diseases (IDDs), sometimes called Idiopathic (IIDDs) due to the unknown etiology of some of them, are a heterogenous group of demyelinating diseases - conditions that cause damage to myelin, the protective sheath of nerve fibers - that occur against the background of an acute or chronic inflammatory process. IDDs share characteristics with and are often grouped together under Multiple Sclerosis. They are sometimes considered different diseases from Multiple Sclerosis, but considered by others to form a spectrum differing only in terms of chronicity, severity, and clinical course.

Baló's concentric sclerosis is a disease in which the white matter of the brain appears damaged in concentric layers, leaving the axis cylinder intact. It was described by József Mátyás Baló who initially named it "leuko-encephalitis periaxialis concentrica" from the previous definition, and it is currently considered one of the borderline forms of multiple sclerosis.
Marburg acute multiple sclerosis, also known as Marburg multiple sclerosis or acute fulminant multiple sclerosis, is considered one of the multiple sclerosis borderline diseases, which is a collection of diseases classified by some as MS variants and by others as different diseases. Other diseases in this group are neuromyelitis optica (NMO), Balo concentric sclerosis, and Schilder's disease. The graver course is one form of malignant multiple sclerosis, with patients reaching a significant level of disability in less than five years from their first symptoms, often in a matter of months.
Eugène Devic was a French neurologist who was a native of Lyon.
Research in multiple sclerosis may find new pathways to interact with the disease, improve function, curtail attacks, or limit the progression of the underlying disease. Many treatments already in clinical trials involve drugs that are used in other diseases or medications that have not been designed specifically for multiple sclerosis. There are also trials involving the combination of drugs that are already in use for multiple sclerosis. Finally, there are also many basic investigations that try to understand better the disease and in the future may help to find new treatments.

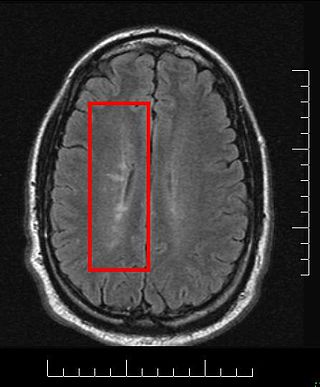
Tumefactive multiple sclerosis is a condition in which the central nervous system of a person has multiple demyelinating lesions with atypical characteristics for those of standard multiple sclerosis (MS). It is called tumefactive as the lesions are "tumor-like" and they mimic tumors clinically, radiologically and sometimes pathologically.
Chronic relapsing inflammatory optic neuropathy (CRION) is a form of recurrent optic neuritis that is steroid responsive and dependent. Patients typically present with pain associated with visual loss. CRION is a clinical diagnosis of exclusion, and other demyelinating, autoimmune, and systemic causes should be ruled out. An accurate antibody test which became available commercially in 2017 has allowed most patients previously diagnosed with CRION to be re-identified as having MOG antibody disease, which is not a diagnosis of exclusion. Early recognition is crucial given risks for severe visual loss and because it is treatable with immunosuppressive treatment such as steroids or B-cell depleting therapy. Relapse that occurs after reducing or stopping steroids is a characteristic feature.
MOG antibody disease (MOGAD) or MOG antibody-associated encephalomyelitis (MOG-EM) is an inflammatory demyelinating disease of the central nervous system. Serum anti-myelin oligodendrocyte glycoprotein antibodies are present in up to half of patients with an acquired demyelinating syndrome and have been described in association with a range of phenotypic presentations, including acute disseminated encephalomyelitis, optic neuritis, transverse myelitis, and neuromyelitis optica.
Inebilizumab, sold under the brand name Uplizna, is a medication for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adults. Inebilizumab is a humanized mAb that binds to and depletes CD19+ B cells including plasmablasts and plasma cells.
Satralizumab, sold under the brand name Enspryng, is a humanized monoclonal antibody medication that is used for the treatment of neuromyelitis optica spectrum disorder (NMOSD), a rare autoimmune disease. The drug is being developed by Chugai Pharmaceutical, a subsidiary of Roche.
Anti-neurofascin demyelinating diseases refers to health conditions engendered by auto-antibodies against neurofascins, which can produce both central and peripheral demyelination. Some cases of combined central and peripheral demyelination (CCPD) could be produced by them.










