Related Research Articles

In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. The term antigen originally referred to a substance that is an antibody generator. Antigens can be proteins, peptides, polysaccharides, lipids, or nucleic acids.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
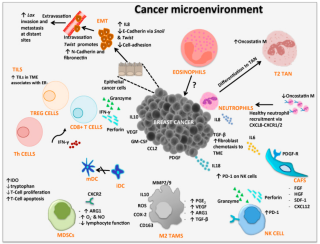
Cancer immunology is an interdisciplinary branch of biology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.
Vaccine therapy is a type of treatment that uses a substance or group of substances to stimulate the immune system to destroy a tumor or infectious microorganisms such as bacteria or viruses.
CancerVax was an American pharmaceutical company founded in 1998 by Donald Morton. The company sought to develop a vaccine for cancer, and had candidates for melanoma reach phase III clinical trials. When those trials proved unsuccessful in 2005, the company soon underwent a reverse takeover with Micromet.
James L. Gulley is an American cancer researcher and the Director of the Medical Oncology Service at National Cancer Institute.
Dendreon is a biotechnology company. Its lead product, Provenge, is an immunotherapy for prostate cancer. It consists of a mixture of the patient's own blood cells that have been incubated with the Dendreon PAP-GM-CSF fusion protein. Phase III clinical trial results demonstrating a survival benefit for prostate cancer patients receiving the drug were presented at the AUA meeting on April 28, 2009. After going through the approval process, Provenge was given full approval by the FDA on April 29, 2010. Dendreon's stock value fell 66% on August 4, 2011, after abandoning its forecast for its debut drug Provenge.
Sipuleucel-T, sold under the brand name Provenge, developed by Dendreon Pharmaceuticals, LLC, is a cell-based cancer immunotherapy for prostate cancer (CaP). It is an autologous cellular immunotherapy.
Neuvenge, Lapuleucel-T, is a therapeutic cancer vaccine (TCV) in development by Dendreon (DNDN). It uses the "immunotherapy platform approach" first successfully demonstrated on the U.S. Food and Drug Administration (FDA)-approved TCV Provenge. It was first tested on breast cancer patients with tumors expressing HER2/neu, and is now scheduled to be tested on bladder cancer patients.
ALECSAT technology is a novel method of epigenetic cancer immunotherapy being used by the company CytoVac. It uses a patient's own immune system to target tumor cells in prostate cancer, glioblastomas, and potentially pancreatic cancer. ALECSAT research, directed by Alexei Kirken and Karine Dzhandzhugazyan, has led to several clinical trials.

James Patrick Allison is an American immunologist and Nobel laureate who holds the position of professor and chair of immunology and executive director of immunotherapy platform at the MD Anderson Cancer Center at the University of Texas.
PROSTVAC is a cancer immunotherapy candidate in clinical development by Bavarian Nordic for the treatment of all prostate cancer although clinical trials are focusing on more advanced cases of metastatic castration-resistant prostate cancer (mCRPC). PROSTVAC is a vaccine designed to enable the immune system to recognize and attack prostate cancer cells by triggering a specific and targeted T cell immune response to cancer cells that express the tumor-associated antigen prostate-specific antigen (PSA).
Eftilagimod alpha is a large-molecule cancer drug being developed by the clinical-stage biotechnology company Immutep. Efti is a soluble version of the immune checkpoint molecule LAG-3. It is an APC Activator used to increase an immune response to tumors, and is administered by subcutaneous injection. Efti has three intended clinical settings:
The dendritic cell-based cancer vaccine is an innovation in therapeutic strategy for cancer patients.
The Immune Response Corporation (IRC) pioneered immunotherapeutic products. The firm was founded by Jonas Salk and Kevin Kimberlin when Kimberlin, "asked Salk to become lead scientific advisor for a new biotech company specializing in 'anti-idiotypes,' a novel vaccine technology," Salk called the proposal "liberating."
A therapeutic vaccine is a vaccine which is administered after a disease or infection has already occurred. A therapeutic vaccine works by activating the immune system of a patient to fight an infection. A therapeutic vaccine differs from a prophylactic vaccine in that prophylactic vaccines are administered to individuals as a precautionary measure to avoid the infection or disease while therapeutic vaccines are administered after the individual is already affected by the disease or infection. A therapeutic vaccine fights an existing infection in the body rather than immunizing the body for protection against future diseases and infections. Therapeutic vaccines are mostly used against viral infections. Patients affected with chronic viral infections are administered with therapeutic vaccines, as their immune system is not able to produce enough efficient antibodies.

Claudia M. Palena is an Argentine-American immunologist and cancer researcher. She is head of the immunoregulation section at the National Cancer Institute. Palena researches tumor immunology and cancer immunotherapy.
References
- ↑ O'Neill, David W. (November 2010). "Dendritic cells and T cells in immunotherapy". Journal of Drugs in Dermatology: JDD. 9 (11): 1383–1392. ISSN 1545-9616. PMID 21061761.
- ↑ MD, Marc S. Ernstoff; FACP, Igor Puzanov, MD, MSCI; PhD, Caroline Robert, MD; MD, Adi M. Diab; PhD, Peter M. Hersey, MD (2019-03-15). SITC's Guide to Managing Immunotherapy Toxicity. Springer Publishing Company. pp. xviii. ISBN 978-0-8261-7215-0.