
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry.

Arsine (IUPAC name: arsane) is an inorganic compound with the formula AsH3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term arsine is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine".

Arsenic trisulfide is the inorganic compound with the formula As2S3. It is a dark yellow solid that is insoluble in water. It also occurs as the mineral orpiment, which has been used as a pigment called King's yellow. It is produced in the analysis of arsenic compounds. It is a group V/VI, intrinsic p-type semiconductor and exhibits photo-induced phase-change properties.
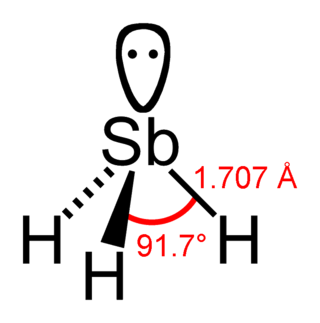
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). This gas has an offensive smell like hydrogen sulfide (rotten eggs).

Arsenic trioxide, sold under the brand name Trisenox among others, is an inorganic compound with the formula As
2O
3. As an industrial chemical, its major uses include the manufacture of wood preservatives, pesticides, and glass. It is also used as a medication to treat a type of cancer known as acute promyelocytic leukemia. For this use it is given by injection into a vein.

Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions with hydrolysis. A mixed arsenic-antimony oxide occurs in nature as the very rare mineral stibioclaudetite.

Arsenous acid (or arsenious acid) is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3.

Chromium(III) fluoride is the name for the inorganic compounds with the chemical formula CrF3 as well as several related hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the coloured hydrates [Cr(H2O)6]F3 and [Cr(H2O)6]F3•3H2O are soluble in water. The trihydrate is green, and the hexahydrate is violet. The anhydrous form sublimes at 1100–1200 °C.
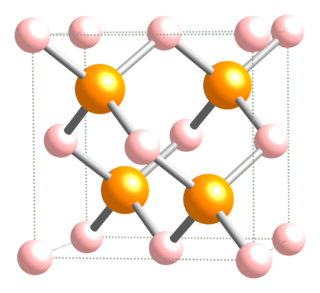
Aluminium arsenide is a semiconductor material with almost the same lattice constant as gallium arsenide and aluminium gallium arsenide and wider band gap than gallium arsenide. (AlAs) can form a superlattice with gallium arsenide (GaAs) which results in its semiconductor properties. Because GaAs and AlAs have almost the same lattice constant, the layers have very little induced strain, which allows them to be grown almost arbitrarily thick. This allows for extremely high performance high electron mobility, HEMT transistors, and other quantum well devices.
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

Sodium arsenite usually refers to the inorganic compound with the formula NaAsO2. Also called sodium meta-arsenite, it is the sodium salt of arsenous acid. Sodium ortho-arsenite is Na3AsO3. The compounds are colourless solids.

Scheele's Green, also called Schloss Green, is chemically a cupric hydrogen arsenite, CuHAsO
3. It is chemically related to Paris Green. Scheele's Green was invented in 1775 by Carl Wilhelm Scheele. By the end of the 19th century, it had virtually replaced the older green pigments based on copper carbonate. It is a yellowish-green pigment commonly used during the early to mid-19th century in paints as well as being directly incorporated into a variety of products as a colorant. It began to fall out of favor after the 1860s because of its toxicity and the instability of its color in the presence of sulfides and various chemical pollutants. The acutely toxic nature of Scheele's green as well as other arsenic-containing green pigments such as Paris Green may have contributed to the sharp decline in the popularity of the color green in late Victorian society. By the dawn of the 20th century, Scheele's green had completely fallen out of use as a pigment but was still in use as an insecticide into the 1930s. At least two modern reproductions of Scheele's green hue with modern non-toxic pigments have been made, with similar but non-identical color coordinates: one with hex#3c7a18 and another with hex#478800. The latter is the more typically reported color coordinate for Scheele's green.

Arsenic tribromide is the inorganic compound with the formula AsBr3. This pyramidal molecule is the only known binary arsenic bromide. AsBr3 is noteworthy for its very high refractive index of approximately 2.3. It also has a very high diamagnetic susceptibility. The compound exists as colourless deliquescent crystals that fume in moist air.

Arsenic trichloride is an inorganic compound with the formula AsCl3, also known as arsenous chloride or butter of arsenic. This poisonous oil is colourless, although impure samples may appear yellow. It is an intermediate in the manufacture of organoarsenic compounds.

Calcium arsenate is the inorganic compound with the formula Ca3(AsO4)2. A colourless salt, it was originally used as a pesticide and as a germicide. It is highly soluble in water, in contrast to lead arsenate, which makes it more toxic. The minerals rauenthalite Ca3(AsO4)2·10H2O and phaunouxite Ca3(AsO4)2·11H2O are hydrates of calcium arsenate.
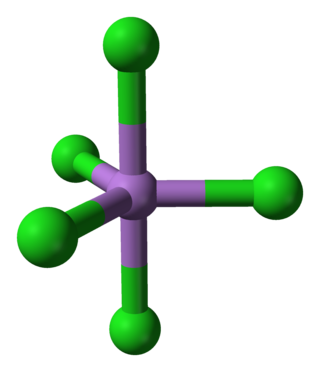
Arsenic pentachloride is a chemical compound of arsenic and chlorine. This compound was first prepared in 1976 through the UV irradiation of arsenic trichloride, AsCl3, in liquid chlorine at −105 °C. AsCl5 decomposes at around −50 °C. The structure of the solid was finally determined in 2001. AsCl5 is similar to phosphorus pentachloride, PCl5 in having a trigonal bipyramidal structure where the equatorial bonds are shorter than the axial bonds (As-Cleq = 210.6 pm, 211.9 pm; As-Clax= 220.7 pm).
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water.
Arsenic pentafluoride is a chemical compound of arsenic and fluorine. It is a toxic, colorless gas. The oxidation state of arsenic is +5.

Antimony sulfate, Sb2(SO4)3, is a hygroscopic salt formed by reacting antimony or its compounds with hot sulfuric acid. It is used in doping of semiconductors and in the production of explosives and fireworks.
Ammonium orthomolybdate is the inorganic compound with the chemical formula (NH4)2MoO4. It is a white solid that is prepared by treating molybdenum trioxide with aqueous ammonia. Upon heating these solutions, ammonia is lost, to give ammonium heptamolybdate ((NH4)6Mo7O24·4H2O).

















