Related Research Articles
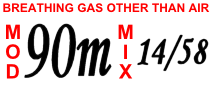
Narcosis while diving is a reversible alteration in consciousness that occurs while diving at depth. It is caused by the anesthetic effect of certain gases at high pressure. The Greek word νάρκωσις (narkōsis), "the act of making numb", is derived from νάρκη (narkē), "numbness, torpor", a term used by Homer and Hippocrates. Narcosis produces a state similar to drunkenness, or nitrous oxide inhalation. It can occur during shallow dives, but does not usually become noticeable at depths less than 30 meters (100 ft).
Asphyxia or asphyxiation is a condition of deficient supply of oxygen to the body which arises from abnormal breathing. Asphyxia causes generalized hypoxia, which affects primarily the tissues and organs. There are many circumstances that can induce asphyxia, all of which are characterized by the inability of a person to acquire sufficient oxygen through breathing for an extended period of time. Asphyxia can cause coma or death.
An inert gas is a gas that does not form chemical reaction with another chemical substance and therefore does not form chemical compounds. The noble gases often do not react with many substances and were historically referred to as the inert gases. Inert gases are used generally to avoid unwanted chemical reactions degrading a sample. These undesirable chemical reactions are often oxidation and hydrolysis reactions with the oxygen and moisture in air. The term inert gas is context-dependent because several of the noble gases can be made to react under certain conditions.

A rebreather is a breathing apparatus that absorbs the carbon dioxide of a user's exhaled breath to permit the rebreathing (recycling) of the substantially unused oxygen content, and unused inert content when present, of each breath. Oxygen is added to replenish the amount metabolised by the user. This differs from open-circuit breathing apparatus, where the exhaled gas is discharged directly into the environment. The purpose is to extend the breathing endurance of a limited gas supply, and, for covert military use by frogmen or observation of underwater life, eliminating the bubbles produced by an open circuit system. A rebreather is generally understood to be a portable unit carried by the user. The same technology on a vehicle or non-mobile installation is more likely to be referred to as a life-support system.

A breathing gas is a mixture of gaseous chemical elements and compounds used for respiration. Air is the most common and only natural breathing gas, but other mixtures of gases, or pure oxygen, are also used in breathing equipment and enclosed habitats such as scuba equipment, surface supplied diving equipment, recompression chambers, high-altitude mountaineering, high-flying aircraft, submarines, space suits, spacecraft, medical life support and first aid equipment, and anaesthetic machines.

Hypercapnia (from the Greek hyper = "above" or "too much" and kapnos = "smoke"), also known as hypercarbia and CO2 retention, is a condition of abnormally elevated carbon dioxide (CO2) levels in the blood. Carbon dioxide is a gaseous product of the body's metabolism and is normally expelled through the lungs. Carbon dioxide may accumulate in any condition that causes hypoventilation, a reduction of alveolar ventilation (the clearance of air from the small sacs of the lung where gas exchange takes place) as well as resulting from inhalation of CO2. Inability of the lungs to clear carbon dioxide, or inhalation of elevated levels of CO2, leads to respiratory acidosis. Eventually the body compensates for the raised acidity by retaining alkali in the kidneys, a process known as "metabolic compensation".

Bottled gas is a term used for substances which are gaseous at standard temperature and pressure (STP) and have been compressed and stored in carbon steel, stainless steel, aluminum, or composite bottles known as gas cylinders.

A respirator is a device designed to protect the wearer from inhaling hazardous atmospheres, including fumes, vapours, gases and particulate matter such as dusts and airborne pathogens such as viruses. There are two main categories: the air-purifying respirator, in which respirable air is obtained by filtering a contaminated atmosphere, and the air-supplied respirator, in which an alternate supply of breathable air is delivered. Within each category, different techniques are employed to reduce or eliminate noxious airborne contaminants.

A confined space is a space with limited entry and egress and not suitable for human inhabitants. An example is the interior of a storage tank, occasionally entered by maintenance workers but not intended for human occupancy. Hazards in a confined space often include harmful dust or gases, asphyxiation, submersion in liquids or free-flowing granular solids, electrocution, or entrapment.

The term immediately dangerous to life or health (IDLH) is defined by the US National Institute for Occupational Safety and Health (NIOSH) as exposure to airborne contaminants that is "likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from such an environment." Examples include smoke or other poisonous gases at sufficiently high concentrations. It is calculated using the LD50 or LC50. The Occupational Safety and Health Administration (OSHA) regulation defines the term as "an atmosphere that poses an immediate threat to life, would cause irreversible adverse health effects, or would impair an individual's ability to escape from a dangerous atmosphere."
Shielding gases are inert or semi-inert gases that are commonly used in several welding processes, most notably gas metal arc welding and gas tungsten arc welding. Their purpose is to protect the weld area from oxygen, and water vapour. Depending on the materials being welded, these atmospheric gases can reduce the quality of the weld or make the welding more difficult. Other arc welding processes use alternative methods of protecting the weld from the atmosphere as well – shielded metal arc welding, for example, uses an electrode covered in a flux that produces carbon dioxide when consumed, a semi-inert gas that is an acceptable shielding gas for welding steel.
Degassing, also known as degasification, is the removal of dissolved gases from liquids, especially water or aqueous solutions. There are numerous methods for removing gases from liquids.
Diving disorders, or diving related medical conditions, are conditions associated with underwater diving, and include both conditions unique to underwater diving, and those that also occur during other activities. This second group further divides into conditions caused by exposure to ambient pressures significantly different from surface atmospheric pressure, and a range of conditions caused by general environment and equipment associated with diving activities.
Inert gas asphyxiation is a form of asphyxiation which results from breathing a physiologically inert gas in the absence of oxygen, or a low amount of oxygen, rather than atmospheric air. Examples of physiologically inert gases, which have caused accidental or deliberate death by this mechanism, are argon, helium, nitrogen and methane. The term "physiologically inert" is used to indicate a gas which has no toxic or anesthetic properties and does not act upon the heart or hemoglobin. Instead, the gas acts as a simple diluent to reduce oxygen concentration in inspired gas and blood to dangerously low levels, thereby eventually depriving all cells in the body of oxygen.
Blackdamp is an asphyxiant, reducing the available oxygen content of air to a level incapable of sustaining human or animal life. It is not a single gas but a mixture of unbreathable gases left after oxygen is removed from the air and typically consists of nitrogen, carbon dioxide and water vapour. The term is etymologically and practically related to terms for other underground mine gases such as fire damp, white damp, and stink damp, and afterdamp.

Manure management refers to capture, storage, treatment, and utilization of animal manures in an environmentally sustainable manner. It can be retained in various holding facilities. Animal manure can occur in a liquid, slurry, or solid form. It is utilized by distribution on fields in amounts that enrich soils without causing water pollution or unacceptably high levels of nutrient enrichment. Manure management is a component of nutrient management.
A suicide bag, also known as an exit bag or hood, is part of a euthanasia device consisting of a large plastic bag with a drawcord used to commit suicide through inert gas asphyxiation. It is usually used in conjunction with a flow of an inert gas that is lighter or less dense than air, like helium or nitrogen, which prevents the panic, sense of suffocation and struggling before unconsciousness, known as the hypercapnic alarm response caused by the presence of high carbon dioxide concentrations in the blood. This method also makes the direct cause of death difficult to trace if the bag and gas canister are removed before the death is reported. While asphyxiation by helium can be detected at autopsy, there is currently no test that can detect asphyxiation by nitrogen. For this reason, nitrogen is commonly the preferred choice for people who do not want the cause of death established.
Gas blending is the process of mixing gases for a specific purpose where the composition of the resulting mixture is specified and controlled. A wide range of applications include scientific and industrial processes, food production and storage and breathing gases.

The physiology of decompression involves a complex interaction of gas solubility, partial pressures and concentration gradients, diffusion, bulk transport and bubble mechanics in living tissues. Gas is breathed at ambient pressure, and some of this gas dissolves into the blood and other fluids. Inert gas continues to be taken up until the gas dissolved in the tissues is in a state of equilibrium with the gas in the lungs,, or the ambient pressure is reduced until the inert gases dissolved in the tissues are at a higher concentration than the equilibrium state, and start diffusing out again.
Human physiology of underwater diving is the physiological influences of the underwater environment on the human diver, and adaptations to operating underwater, both during breath-hold dives and while breathing at ambient pressure from a suitable breathing gas supply. It, therefore, includes the range of physiological effects generally limited to human ambient pressure divers either freediving or using underwater breathing apparatus. Several factors influence the diver, including immersion, exposure to the water, the limitations of breath-hold endurance, variations in ambient pressure, the effects of breathing gases at raised ambient pressure, effects caused by the use of breathing apparatus, and sensory impairment. All of these may affect diver performance and safety.
References
- 1 2 Terazawa K, Takatori T, Tomii S, Nakano K. Methane asphyxia. Coal mine accident investigation of distribution of gas. Am J Forensic Med Pathol. 1985 Sep;6(3):211-4. PMID 3870672
- ↑ Discussion of the Kursk disaster and death on submarines
- ↑ Kirk JC. Proposed minimum requirements for the operational characteristics and testing of submersible atmosphere monitoring and control units. Life Support Biosph Sci. 1998;5(3):287-94. PMID 11876195
- 1 2 Gill JR, Ely SF, Hua Z.Environmental gas displacement: three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002 Mar;23(1):26-30. PMID 11953489
- ↑ Sahli BP, Armstrong CW.Confined space fatalities in Virginia. J Occup Med. 1992 Sep;34(9):910-7. PMID 1447597
- ↑ BBC article on the Lake Nyos incident
- ↑ Yoshitome K, Ishikawa T, Inagaki S, Yamamoto Y, Miyaishi S, Ishizu H. A case of suffocation by an advertising balloon filled with pure helium gas. Acta Med Okayama. 2002 Feb;56(1):53-5. PMID 11873946
- ↑ OSHA article on asphyxiant gases accidentally fed into respirators
- ↑ Gallagher KE, Smith DM, Mellen PF. Suicidal asphyxiation by using pure helium gas: case report, review, and discussion of the influence of the internet. Am J Forensic Med Pathol. 2003 Dec;24(4):361-3. PMID 14634476
- ↑ Gilson T, Parks BO, Porterfield CM. Suicide with inert gases: addendum to Final Exit. Am J Forensic Med Pathol. 2003 Sep;24(3):306-8. PMID 12960671
- ↑ Shields LB, Hunsaker DM, Hunsaker JC 3rd, Wetli CV, Hutchins KD, Holmes RM. Atypical autoerotic death: part II. Am J Forensic Med Pathol. 2005 Mar;26(1):53-62. PMID 15725777
- ↑ BBC Family of 'helium death' teen warn of inhalation
- ↑ NIOSH [1987a]. NIOSH guide to industrial respiratory protection. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 87-116.
- ↑ OSHA page for nitrogen, a representative asphyxiant gas Archived October 6, 2006, at the Wayback Machine
- ↑ "Publication Detail". Archived from the original on 2006-10-16. Retrieved 2006-10-12. Link to pamphlet SB-2
- ↑ "Publication Detail". Archived from the original on 2006-10-16. Retrieved 2006-10-12. Link to pamphlet SB-28
- ↑ "CGA Position on Oderizing". Archived from the original on 2007-09-28. Retrieved 2006-10-12. Summary of CGA position on odorizing. Accessed 10/11/06
- ↑ "Publication Detail". Archived from the original on 2006-10-16. Retrieved 2006-10-12. Full text of CGA position on odorizing. Accessed 10/11/06
- ↑ "Mine Safety and Health Administration article about mine fire survival. Accessed 10/12/06". Archived from the original on 2006-10-09. Retrieved 2006-10-13.
- ↑ "MSHA copy of the Mine Act of 1977. Accessed 10/12/06". Archived from the original on 2015-09-05. Retrieved 2006-10-13.