
A toxin is a naturally occurring organic poison produced by metabolic activities of living cells or organisms. They occur especially as proteins, often conjugated. The term was first used by organic chemist Ludwig Brieger (1849–1919) and is derived from the word "toxic".

Mussel is the common name used for members of several families of bivalve molluscs, from saltwater and freshwater habitats. These groups have in common a shell whose outline is elongated and asymmetrical compared with other edible clams, which are often more or less rounded or oval.

Tetrodotoxin (TTX) is a potent neurotoxin. Its name derives from Tetraodontiformes, an order that includes pufferfish, porcupinefish, ocean sunfish, and triggerfish; several of these species carry the toxin. Although tetrodotoxin was discovered in these fish, it is found in several other animals. It is also produced by certain infectious or symbiotic bacteria like Pseudoalteromonas, Pseudomonas, and Vibrio as well as other species found in symbiotic relationships with animals and plants.
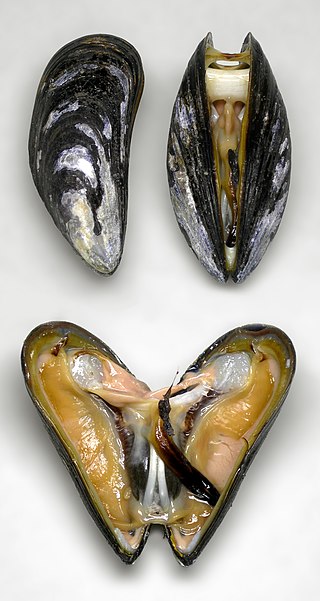
The blue mussel, also known as the common mussel, is a medium-sized edible marine bivalve mollusc in the family Mytilidae, the mussels. Blue mussels are subject to commercial use and intensive aquaculture. A species with a large range, empty shells are commonly found on beaches around the world.

Saxitoxin (STX) is a potent neurotoxin and the best-known paralytic shellfish toxin (PST). Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic algal blooms, is responsible for the illness known as paralytic shellfish poisoning (PSP).

Palytoxin, PTX or PLTX is an intense vasoconstrictor, and is considered to be one of the most poisonous non-protein substances known, second only to maitotoxin in terms of toxicity in mice.
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. A polyketide, polyether derivative of a C38 fatty acid, okadaic acid and other members of its family have shined light upon many biological processes both with respect to dinoflagellete polyketide synthesis as well as the role of protein phosphatases in cell growth.

Paralytic shellfish poisoning (PSP) is one of the four recognized syndromes of shellfish poisoning, which share some common features and are primarily associated with bivalve mollusks. These shellfish are filter feeders and accumulate neurotoxins, chiefly saxitoxin, produced by microscopic algae, such as dinoflagellates, diatoms, and cyanobacteria. Dinoflagellates of the genus Alexandrium are the most numerous and widespread saxitoxin producers and are responsible for PSP blooms in subarctic, temperate, and tropical locations. The majority of toxic blooms have been caused by the morphospecies Alexandrium catenella, Alexandrium tamarense, Gonyaulax catenella and Alexandrium fundyense, which together comprise the A. tamarense species complex. In Asia, PSP is mostly associated with the occurrence of the species Pyrodinium bahamense.
Amnesic shellfish poisoning (ASP) is an illness caused by consumption of shellfish that contain the marine biotoxin called domoic acid. In mammals, including humans, domoic acid acts as a neurotoxin, causing permanent short-term memory loss, brain damage, and death in severe cases.

The California mussel is a large edible mussel, a marine bivalve mollusk in the family Mytilidae.

The Chilean mussel or Chilean blue mussel is a species of blue mussel native to the coasts of Chile from Biobío Region to Cape Horn. Today genomic evidence confirmed that the native Chilean blue mussel is genetically distinct from the Northern Hemisphere M. edulis, M. galloprovincialis and M. trossulus and also genetically different from Mytilus platensis,the other species of smooth shelled mussel from South America.

Leukoma staminea, commonly known as the Pacific littleneck clam, the littleneck clam, the rock cockle, the hardshell clam, the Tomales Bay cockle, the rock clam or the ribbed carpet shell, is a species of bivalve mollusc in the family Veneridae. This species of mollusc was exploited by early humans in North America; for example, the Chumash peoples of Central California harvested these clams in Morro Bay approximately 1,000 years ago, and the distinctive shells form middens near their settlements.

Mytilus is a cosmopolitan genus of medium to large-sized edible, mainly saltwater mussels, marine bivalve molluscs in the family Mytilidae.
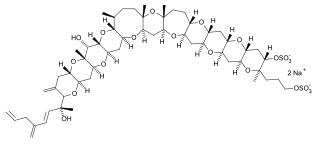
Yessotoxins are a group of lipophilic, sulfur bearing polyether toxins that are related to ciguatoxins. They are produced by a variety of dinoflagellates, most notably Lingulodinium polyedrum and Gonyaulax spinifera.

Saxidomus gigantea is a large, edible saltwater clam, a marine bivalve mollusk in the family Veneridae, the venus clams. It can be found along the western coast of North America, ranging from the Aleutian Islands to San Francisco Bay. Common names for this clam include butter clam, Washington clam, smooth Washington clam and money shell.
Canadian Reference Materials (CRM) are certified reference materials of high-quality and reliability produced by the National Metrology Institute of Canada – the National Research Council Canada. The NRC Certified Reference Materials program is operated by the Measurement Science and Standards portfolio and provides CRMs for environmental, biotoxin, food, nutritional supplement, and stable isotope analysis. The program was established in 1976 to produce CRMs for inorganic and organic marine environmental analysis and remains internationally recognized producer of CRMs.
Azadinium spinosum is a species of dinoflagellates that produces azaspiracid toxins, particularly AZA 1, AZA 2 and an isomer of AZA 2.

Dinophysis acuta is a species of flagellated planktons belonging to the genus Dinophysis. It is one of the few unusual photosynthetic protists that acquire plastids from algae by endosymbiosis. By forming massive blooms, particularly in late summer and spring, it causes red tides. It produces toxic substances and the red tides cause widespread infection of seafood, particularly crabs and mussels. When infected animals are consumed, severe diarrhoea occurs. The clinical symptom is called diarrhetic shellfish poisoning. The main chemical toxins were identified in 2006 as okadaic acid and pectenotoxins. They can produce non-fatal or fatal amounts of toxins in their predators, which can become toxic to humans.

Decarbamoylsaxitoxin, abbreviated as dcSTX, is a neurotoxin which is naturally produced in dinoflagellate. DcSTX is one of the many analogues of saxitoxin (STX).
Prymnesin-2 is an organic compound that is secreted by the haptophyte Prymnesium parvum. It belongs to the prymnesin family and has potent hemolytic and ichthyotoxic properties. In a purified form it appears as a pale yellow solid. P. parvum is responsible for red harmful algal blooms worldwide, causing massive fish killings. When these algal blooms occur, this compound poses a threat to the local fishing industry. This is especially true for brackish water, as the compound can reach critical concentrations more easily.














