
The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (np) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons.

Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element.

An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, opacity, and luster, but may have properties that differ from those of the pure metals, such as increased strength or hardness. In some cases, an alloy may reduce the overall cost of the material while preserving important properties. In other cases, the mixture imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength.
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during reactions with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds.

A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described has having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding.
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.

A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.

The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows ("periods") and columns ("groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics.

In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes:

The mole (symbol mol) is the unit of measurement for amount of substance, a quantity proportional to the number of elementary entities of a substance. It is a base unit in the International System of Units (SI). One mole contains exactly 6.02214076×1023 elementary entities (approximately 602 sextillion or 602 billion times a trillion), which can be atoms, molecules, ions, or other particles. The number of particles in a mole is the Avogadro number (symbol N0) and the numerical value of the Avogadro constant (symbol NA) expressed in mol-1. The value was chosen based on the historical definition of the mole as the amount of substance that corresponds to the number of atoms in 12 grams of 12C, which made the mass of a mole of a compound expressed in grams numerically equal to the average molecular mass of the compound expressed in daltons. With the 2019 redefinition of the SI base units, the numerical equivalence is now only approximate but may be assumed for all practical purposes.
Captain Atom is the name of several superheroes appearing in American comic books, initially owned by Charlton Comics before being acquired in the 1980s by DC Comics. All possess some form of energy-manipulating abilities.
The name Atom applies to a pair of related Web standards. The Atom Syndication Format is an XML language used for web feeds, while the Atom Publishing Protocol is a simple HTTP-based protocol for creating and updating web resources.
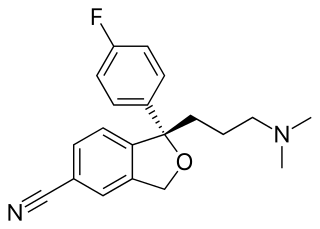
The skeletal formula, line-angle formula, or shorthand formula of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A skeletal formula shows the skeletal structure or skeleton of a molecule, which is composed of the skeletal atoms that make up the molecule. It is represented in two dimensions, as on a piece of paper. It employs certain conventions to represent carbon and hydrogen atoms, which are the most common in organic chemistry.

Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.
In chemistry, the valence or valency of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemical bonds that each atom of a given chemical element typically forms. Double bonds are considered to be two bonds, triple bonds to be three, quadruple bonds to be four, quintuple bonds to be five and sextuple bonds to be six. In most compounds, the valence of hydrogen is 1, of oxygen is 2, of nitrogen is 3, and of carbon is 4. Valence is not to be confused with the related concepts of the coordination number, the oxidation state, or the number of valence electrons for a given atom.

The Rutherford model was devised by Ernest Rutherford to describe an atom. Rutherford directed the Geiger–Marsden experiment in 1909, which suggested, upon Rutherford's 1911 analysis, that J. J. Thomson's plum pudding model of the atom was incorrect. Rutherford's new model for the atom, based on the experimental results, contained new features of a relatively high central charge concentrated into a very small volume in comparison to the rest of the atom and with this central volume containing most of the atom's mass; this region would be known as the atomic nucleus. The Rutherford model was subsequently superseded by the Bohr model.

Intel Atom is a line of IA-32 and x86-64 instruction set ultra-low-voltage processors by Intel Corporation designed to reduce electric consumption and power dissipation in comparison with ordinary processors of the Intel Core series. Atom is mainly used in netbooks, nettops, embedded applications ranging from health care to advanced robotics, mobile Internet devices (MIDs) and phones. The line was originally designed in 45 nm complementary metal–oxide–semiconductor (CMOS) technology and subsequent models, codenamed Cedar, used a 32 nm process.

An ion is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.

A chemical compound is a chemical substance composed of many identical molecules containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed.
Atom is a free and open-source text and source-code editor for macOS, Linux, and Windows with support for plug-ins written in JavaScript, and embedded GitGit control. Developed by GitHub, Atom was released on June 25, 2015.


















