
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.
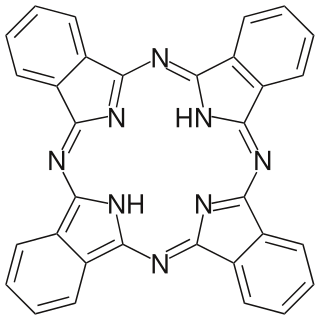
Phthalocyanine is a large, aromatic, macrocyclic, organic compound with the formula (C8H4N2)4H2 and is of theoretical or specialized interest in chemical dyes and photoelectricity.
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are a synthetic dye family belonging to the polymethine group. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV.
Phosphole is the organic compound with the chemical formula C
4H
4PH; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve as ligands for transition metals and as precursors to more complex organophosphorus compounds.

A boronic acid is an organic compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes.

Squaraine dyes are a class of organic dyes showing intense fluorescence, typically in the red and near infrared region. They are characterized by their unique aromatic four membered ring system derived from squaric acid. Most squaraines are encumbered by nucleophilic attack of the central four membered ring, which is highly electron deficient. This encumbrance can be attenuated by the formation of a rotaxane around the dye to protect it from nucleophiles. They are currently used as sensors for ions and have recently, with the advent of protected squaraine derivatives, been exploited in biomedical imaging.

Metalloles are metallacycle derivatives of cyclopentadiene in which the carbon atom at position 5, the saturated carbon, is replaced by a heteroatom. In contrast to its parent compound, the numbering of the metallole starts at the heteroatom. Some of these compounds are described as organometallic compounds, but in the list below quite a number of metalloids are present too. Many metalloles are fluorescent. Polymeric derivatives of pyrrole and thiophene are of interest in molecular electronics. Metalloles, which can also be viewed as structural analogs of pyrrole, include:
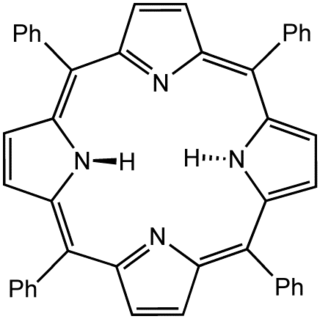
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents. Tetraphenylporphyrin is hydrophobic, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform and benzene.

Indole is an organic compound with the formula C6H4CCNH3. Indoles are derivatives of indole where one or more H's have been replaced by other groups. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.
The Barton–Zard reaction is a route to pyrrole derivatives via the reaction of a nitroalkene with an α-isocyanide under basic conditions. It is named after Derek Barton and Samir Zard who first reported it in 1985.
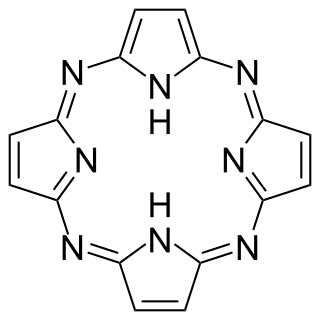
Porphyrazines, or tetraazaporphyrins, are tetrapyrrole macrocycles similar to porphyrins and phthalocyanines. Pioneered by Sir R. Patrick Linstead as an extension of his work on phthalocyanines, porphyrazines differ from porphyrins in that they contain -meso nitrogen atoms, rather than carbon atoms, and differ from phthalocyanines in that their β-pyrrole positions are open for substitution. These differences confer physical properties that are distinct from both porphyrins and phthalocyanines.

Carbon quantum dots also commonly called carbon nano dots are carbon nanoparticles which are less than 10 nm in size and have some form of surface passivation.
Diketopyrrolopyrroles (DPPs) are organic dyes and pigments based on the heterocyclic dilactam 2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione, widely used in optoelectronics. DPPs were initially used as pigments in the painting industry due to their high resistance to photodegradation. More recently, DPP derivatives have been also investigated as promising fluorescent dyes for bioimaging applications, as well as components of materials for use in organic electronics.

1,3-Diphenylisobenzofuran is a highly reactive diene that can scavenge unstable and short-lived dienophiles in a Diels-Alder reaction. It is furthermore used as a standard reagent for the determination of singlet oxygen, even in biological systems. Cycloadditions with 1,3-diphenylisobenzofuran and subsequent oxygen cleavage provide access to a variety of polyaromatics.
Boron porphyrins are a variety of porphyrin, a common macrocycle used for photosensitization and metal trapping applications, that incorporate boron. The central four nitrogen atoms in a porphyrin macrocycle form a unique molecular pocket which is known to accommodate transition metals of various sizes and oxidation states. Due to the diversity of binding modes available to porphyrin, there is a growing interest in introducing other elements into this pocket.

2,2'-Dipyrromethene, often called just dipyrromethene or dipyrrin, is a chemical compound with formula C
9H
8N
2 whose skeleton can be described as two pyrrole rings C
5N connected by a methyne bridge =CH– through their nitrogen-adjacent (position-2) carbons; the remaining bonds being satisfied by hydrogen atoms. It is an unstable compound that is readily attacked by nucleophilic compounds above −40 °C.
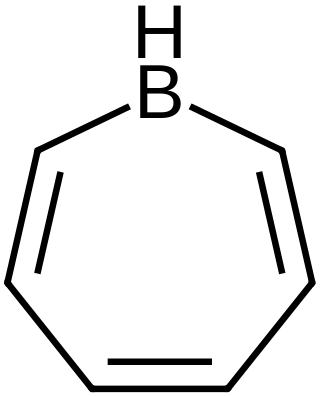
Borepins are a class of boron-containing heterocycles used in main group chemistry. They consist of a seven-membered unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene, which is a seven-membered ring containing three carbon-carbon double bonds, each of which contributes 2π electrons for a total of 6π electrons. Unlike other seven-membered systems such as silepins and phosphepins, boron has a vacant p-orbital that can interact with the π and π* orbitals of the cycloheptatriene. This leads to an isoelectronic state akin to that of the tropylium cation, aromatizing the borepin while also allowing it to act as a Lewis acid. The aromaticity of borepin is relatively weak compared to traditional aromatics such as benzene or even cycloheptatriene, which has led to the synthesis of many fused, π-conjugated borepin systems over the years. Simple and complex borepins have been extensively studied more recently due to their high fluorescence and potential applications in technologies like organic light-emitting diodes (OLEDs) and photovoltaic cells.

Boraacenes are polycyclic aromatic hydrocarbons containing at least one boron atom. Structurally, they are related to acenes, linearly fused benzene rings. However, the boron atom is electron deficient and may act as a Lewis Acid when compared to carbon. This results in slightly less negative charge within the ring, smaller HOMO-LUMO gaps, as well as differences in redox chemistry when compared to their acene analogues. When incorporated into acenes, Boron maintains the planarity and aromaticity of carbon acenes, while adding an empty p-orbital, which can be utilized for the fine tuning of organic semiconductor band gaps. Due to this empty p orbital, however, it is also highly reactive when exposed to nucleophiles like water or normal atmosphere, as it will readily be attacked by oxygen, which must be addressed to maintain its stability.


















