Related Research Articles

Scripps Research, previously known as The Scripps Research Institute (TSRI), is a nonprofit American medical research facility that focuses on research and education in the biomedical sciences. Headquartered in San Diego, California, the institute has over 170 laboratories employing 2,100 scientists, technicians, graduate students, and administrative and other staff, making it the largest private, non-profit biomedical research organization in the United States and among the largest in the world.

Richard Alan Lerner was an American research chemist. He was best known for his work on catalytic antibodies and combinatorial antibody libraries. Lerner served as President of The Scripps Research Institute (TSRI) from 1987 until January 1, 2012, and was a member of its Skaggs Institute for Chemical Biology, in La Jolla, California.

Monoacylglycerol lipase is an enzyme that, in humans, is encoded by the MGLL gene. MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.

Diacylglycerol lipase, also known as DAG lipase, DAGL, or DGL, is an enzyme that catalyzes the hydrolysis of diacylglycerol, releasing a free fatty acid and monoacylglycerol:
diacylglycerol + H2O ⇌ monoacylglycerol + free fatty acid

Fatty acid amide hydrolase or FAAH is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide in 1993. In humans, it is encoded by the gene FAAH.

2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor and the primary endogenous ligand for the CB2 receptor. It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol. It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk. The chemical was first described in 1994-1995, although it had been discovered some time before that. The activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation. 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).

Activity-based proteomics, or activity-based protein profiling (ABPP) is a functional proteomic technology that uses chemical probes that react with mechanistically related classes of enzymes.

AM404, also known as N-arachidonoylaminophenol, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.
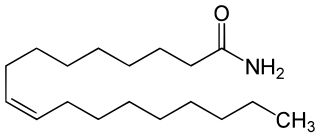
Oleamide is an organic compound with the formula CH3(CH2)7CH=CH(CH2)7CONH2. It is the amide derived from the fatty acid oleic acid. It is a colorless waxy solid and occurs in nature. Sometimes labeled as a fatty acid primary amide (FAPA), it is biosynthesized from N-oleoylglycine.
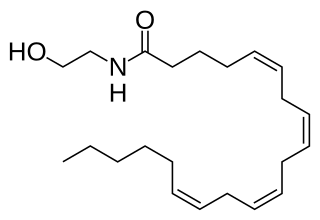
Fatty acid amides (FAAs) are amides formed from a fatty acid and an amine. In nature, many FAAs have ethanolamine as the amine component. Also known as N-acylethanolamines, they contain the functionality RC(O)N(H)CH2CH2OH. A well known example is anandamide. Other fatty acid amides are fatty acid primary amides (FAPAs). They contain the functionality RC(O)NH2). Oleamide is an example of this class of FAPAs.

JZL195 is a potent inhibitor of both fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), the primary enzymes responsible for degrading the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG), respectively.

LY-2183240 is a drug which acts both as a potent inhibitor of the reuptake of the endocannabinoid anandamide and as an inhibitor of fatty acid amide hydrolase (FAAH), the primary enzyme responsible for degrading anandamide. This leads to markedly elevated anandamide levels in the brain, and LY-2183240 has been shown to produce both analgesic and anxiolytic effects in animal models. While LY-2183240 is a potent inhibitor of FAAH, it has relatively poor selectivity and also inhibits several other enzyme side targets. Consequently, it was never developed for clinical use, though it remains widely used in research, and has also been sold as a designer drug.
IDFP is an organophosphorus compound related to the nerve agent sarin.
The endocannabinoid transporters (eCBTs) are transport proteins for the endocannabinoids. Most neurotransmitters are water-soluble and require transmembrane proteins to transport them across the cell membrane. The endocannabinoids on the other hand, are non-charged lipids that readily cross lipid membranes. However, since the endocannabinoids are water immiscible, protein transporters have been described that act as carriers to solubilize and transport the endocannabinoids through the aqueous cytoplasm. These include the heat shock proteins (Hsp70s) and fatty acid-binding proteins for anandamide (FABPs). FABPs such as FABP1, FABP3, FABP5, and FABP7 have been shown to bind endocannabinoids. FABP inhibitors attenuate the breakdown of anandamide by the enzyme fatty acid amide hydrolase (FAAH) in cell culture. One of these inhibitors (SB-FI-26), isolated from a virtual library of a million compounds, belongs to a class of compounds that act as an anti-nociceptive agent with mild anti-inflammatory activity in mice. These truxillic acids and their derivatives have been known to have anti-inflammatory and anti-nociceptive effects in mice and are active components of a Chinese herbal medicine used to treat rheumatism and pain in human. The blockade of anandamide transport may, at least in part, be the mechanism through which these compounds exert their anti-nociceptive effects.

alpha/beta-Hydrolase domain containing 6 (ABHD6), also known as monoacylglycerol lipase ABHD6 or 2-arachidonoylglycerol hydrolase is an enzyme that in humans is encoded by the ABHD6 gene.
N-acylethanolamine acid amide hydrolase (NAAA) EC 3.5.1.- is a member of the choloylglycine hydrolase family, a subset of the N-terminal nucleophile hydrolase superfamily. NAAA has a molecular weight of 31 kDa. The activation and inhibition of its catalytic site is of medical interest as a potential treatment for obesity and chronic pain. While it was discovered within the last decade, its structural similarity to the more familiar acid ceramidase (AC) and functional similarity to fatty acid amide hydrolase (FAAH) allow it to be studied extensively.
PF-3845 is a selective inhibitor of fatty acid amide hydrolase. It results in increased levels of anandamide and results in cannabinoid receptor-based effects. It has anti-inflammatory action in mice colitis models. Antidiarrheal and antinociceptive effects were also seen in mouse models of pain.

alpha/beta-Hydrolase domain containing 12 (ABHD12) is a serine hydrolase encoded by the ABHD12 gene that participates in the breakdown of the endocannabinoid neurotransmitter 2-arachidonylglycerol (2-AG) in the central nervous system. It is responsible for about 9% of brain 2-AG hydrolysis. Together, ABHD12 along with two other enzymes, monoacylglycerol lipase (MAGL) and ABHD6, control 99% of 2-AG hydrolysis in the brain. ABHD12 also serves as a lysophospholipase and metabolizes lysophosphatidylserine (LPS).

Fatty acid amide hydrolase 2 or FAAH2 is a member of the serine hydrolase family of enzymes.

Gary Siuzdak is an American chemist best known for his work in the field of metabolomics, activity metabolomics, and mass spectrometry. His lab discovered indole-3-propionic acid as a gut bacteria derived metabolite in 2009. He is currently the Professor and Director of The Center for Metabolomics and Mass Spectrometry at Scripps Research in La Jolla, California. Siuzdak has also made contributions to virus analysis, viral structural dynamics, as well as developing mass spectrometry imaging technology using nanostructured surfaces. The Siuzdak lab is also responsible for creating the research tools XCMS, METLIN, METLIN Neutral Loss and Q-MRM. As of January 2021, the XCMS/METLIN platform has over 50,000 registered users.
References
- 1 2 3 4 5 6 7 8 9 10 Viegas, Jennifer (2 February 2016). "Profile of Benjamin Cravatt". Proceedings of the National Academy of Sciences of the United States of America . 113 (5): 1109–11. Bibcode:2016PNAS..113.1109V. doi: 10.1073/pnas.1525099113 . PMC 4747733 . PMID 26811454.
- ↑ "National Academy of Sciences Members and Foreign Associates Elected" (Press release). United States: National Academy of Sciences. 29 April 2014. Archived from the original on 18 August 2015. Retrieved 2016-12-31.
Cravatt, Benjamin F.; professor and chair, department of chemical physiology, The Scripps Research Institute, La Jolla, Calif.
- ↑ Bogyo, Matthew; Cravatt, Benjamin F. (February 2007). "Genomics and proteomics: From genes to function: advances in applications of chemical and systems biology". Current Opinion in Chemical Biology (Editorial Overview). 11: 1–3. doi:10.1016/j.cbpa.2006.12.029.
- ↑ Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A. (7 November 1996). "Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides". Nature. 384 (6604): 83–7. Bibcode:1996Natur.384...83C. doi:10.1038/384083a0. PMID 8900284. S2CID 4288981.
- ↑ Cravatt, BF; Prospero-Garcia, O; Siuzdak, G; Gilula, NB; Henriksen, SJ; Boger, DL; Lerner, RA (9 June 1995). "Chemical characterization of a family of brain lipids that induce sleep". Science. 268 (5216): 1506–9. Bibcode:1995Sci...268.1506C. doi:10.1126/science.7770779. PMID 7770779.
- ↑ Wolf Prize in Chemistry 2022