
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. An group of the structure R2C=C=CR− is called allenyl, where R is H or some alkyl group. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding.

In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".
A selenide is a chemical compound containing a selenium with oxidation number of −2. Similar to sulfide, selenides occur both as inorganic compounds and as organic derivatives, which are called organoselenium compound.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols. An exception is the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.

In organic chemistry, nitroso refers to a functional group in which the nitric oxide group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds, S-nitroso compounds, N-nitroso compounds, and O-nitroso compounds.

Hydroperoxides or peroxols are compounds of the form ROOH, where R stands for any group, typically organic, which contain the hydroperoxy functional group. Hydroperoxide also refers to the hydroperoxide anion and its salts, and the neutral hydroperoxyl radical (•OOH) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.
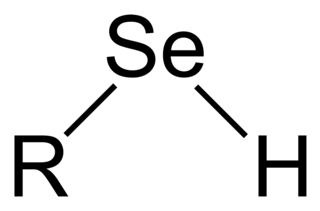
Selenols are organic compounds that contain the functional group with the connectivity C−Se−H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. A well-known selenol is the amino acid selenocysteine.

In organic chemistry, a Grignard reagent or Grignard compound is a chemical compound with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.
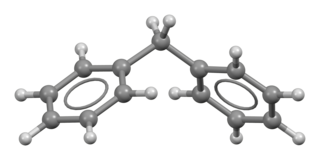
Diphenylmethane is an organic compound with the formula (C6H5)2CH2 (often abbreviated CH
2Ph
2). The compound consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. It is a white solid.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.

Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2. This orange-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis.
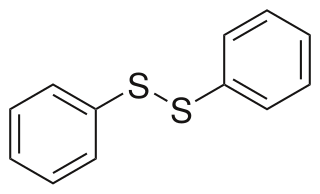
Diphenyl disulfide is the chemical compound with the formula (C6H5S)2. This colorless crystalline material is often abbreviated Ph2S2. It is one of the more commonly encountered organic disulfides in organic synthesis. Minor contamination by thiophenol is responsible for the disagreeable odour associated with this compound.
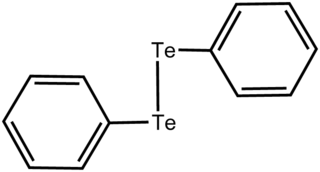
Diphenylditelluride is the chemical compound with the formula (C6H5Te)2, abbreviated Ph2Te2. This orange-coloured solid is the oxidized derivative of the unstable benzenetellurol, PhTeH. Ph2Te2 is used as a source of the PhTe unit in organic synthesis and as a catalyst for redox reactions.

Phenylboronic acid or benzeneboronic acid, abbreviated as PhB(OH)2 where Ph is the phenyl group C6H5-, is a boronic acid containing a phenyl substituent and two hydroxyl groups attached to boron. Phenylboronic acid is a white powder and is commonly used in organic synthesis. Boronic acids are mild Lewis acids which are generally stable and easy to handle, making them important to organic synthesis.
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.
Selenium monochloride or diselenium dichloride is an inorganic compound with the formula Se2Cl2. Although a common name for the compound is selenium monochloride, reflecting its empirical formula, IUPAC does not recommend that name, instead preferring the more descriptive diselenium dichloride.



















