Related Research Articles

Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

An action potential occurs when the membrane potential of a specific cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of excitable cells, which include animal cells like neurons and muscle cells, as well as some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.

Bert Sakmann is a German cell physiologist. He shared the Nobel Prize in Physiology or Medicine with Erwin Neher in 1991 for their work on "the function of single ion channels in cells," and the invention of the patch clamp. Bert Sakmann was Professor at Heidelberg University and is an Emeritus Scientific Member of the Max Planck Institute for Medical Research in Heidelberg, Germany. Since 2008 he leads an emeritus research group at the Max Planck Institute of Neurobiology.

Erwin Neher is a German biophysicist, specializing in the field of cell physiology. For significant contribution in the field, in 1991 he was awarded, along with Bert Sakmann, the Nobel Prize in Physiology or Medicine for "their discoveries concerning the function of single ion channels in cells".

Roderick MacKinnon is an American biophysicist, neuroscientist, and businessman. He is a professor of molecular neurobiology and biophysics at Rockefeller University who won the Nobel Prize in Chemistry together with Peter Agre in 2003 for his work on the structure and operation of ion channels.
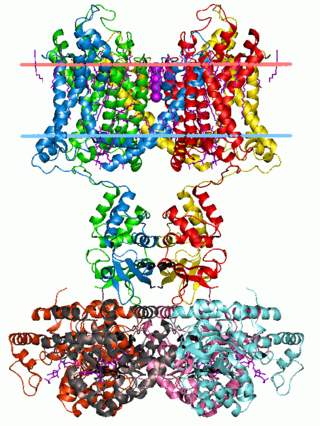
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.
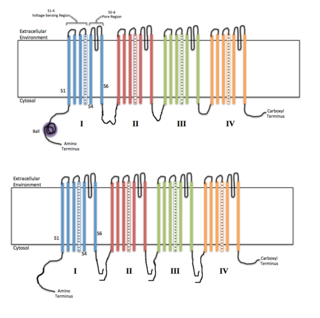
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.
Phosphatidic acids are anionic phospholipids important to cell signaling and direct activation of lipid-gated ion channels. Hydrolysis of phosphatidic acid gives rise to one molecule each of glycerol and phosphoric acid and two molecules of fatty acids. They constitute about 0.25% of phospholipids in the bilayer.

Randy Wayne Schekman is an American cell biologist at the University of California, Berkeley, former editor-in-chief of Proceedings of the National Academy of Sciences and former editor of Annual Review of Cell and Developmental Biology. In 2011, he was announced as the editor of eLife, a new high-profile open-access journal published by the Howard Hughes Medical Institute, the Max Planck Society and the Wellcome Trust launching in 2012. He was elected to the National Academy of Sciences in 1992. Schekman shared the 2013 Nobel Prize for Physiology or Medicine with James Rothman and Thomas C. Südhof for their ground-breaking work on cell membrane vesicle trafficking.
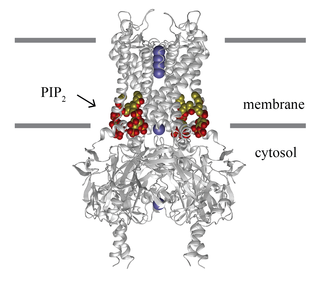
Inward-rectifier potassium channels (Kir, IRK) are a specific lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian and Alan Hodgkin in 1970 in skeletal muscle cells.
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels.
Clay Margrave Armstrong is an American physiologist and a former student of Andrew Fielding Huxley. Armstrong received his MD from Washington University School of Medicine in 1960. He is currently emeritus professor of physiology at the University of Pennsylvania. He has also held professorial appointments at Duke University and the University of Rochester.
Sir Michael John Berridge (22 October 1938 - 13 February 2020) was a British physiologist and biochemist. He was known for his work on cell signaling, in particular the discovery that inositol trisphosphate acts as a second messenger, linking events at the plasma membrane with the release of calcium ions (Ca2+) within the cell.

Julius Bernstein was a German physiologist born in Berlin. His father was Aron Bernstein (1812–1884), a founder of the Reform Judaism Congregation in Berlin 1845; his son was the mathematician Felix Bernstein (1878–1956).
In electrophysiology, the term gating refers to the opening (activation) or closing of ion channels. This change in conformation is a response to changes in transmembrane voltage.
Boris Khodorov was a Soviet and Russian physiologist, M.D., D.Sc., professor of physiology, and head of the Cell Physiology section of Moscow Physiological Society.
Clara Franzini-Armstrong is an Italian-born American electron microscopist, and Professor Emeritus of Cell and Developmental Biology at University of Pennsylvania.
M current is a type of noninactivating potassium current first discovered in bullfrog sympathetic ganglion cells.

Peter Hegemann is a Hertie Senior Research Chair for Neurosciences and a professor of Experimental Biophysics at the Department of Biology, Faculty of Life Sciences, Humboldt University of Berlin, Germany. He is known for his discovery of channelrhodopsin, a type of ion channels regulated by light, thereby serving as a light sensor. This created the field of optogenetics, a technique that controls the activities of specific neurons by applying light. He has received numerous accolades, including the Rumford Prize, the Shaw Prize in Life Science and Medicine, and the Albert Lasker Award for Basic Medical Research.
Sarah K. England is a physiologist and biophysicist and the Alan A. and Edith L. Wolff Professor of Obstetrics and Gynaecology at Washington University School of Medicine. England conducts research on cation channels in uterine smooth muscle to understand the biological correlates of preterm birth and is the Associate Program Director of the Prematurity Research Center at Washington University as well as the Vice Chair of Research for the Center for Reproductive Health Sciences. In 2005, England was selected as a Robert Wood Johnson Foundation Health Policy Fellow in the Office of Senator Hillary Clinton where she used her scientific expertise in obstetrics and gynaecology to guide policy changes.
References
- ↑ "Bertil Hille". University of Washington. Retrieved 10 May 2022.
- 1 2 Aldrich, Richard W. (1 August 2015). "A new standard: A review of Handbook of Ion Channels". Journal of General Physiology. 146 (2): 119–121. doi:10.1085/jgp.201511461. PMC 4516783 . PMID 26216856.
- 1 2 3 Hille, Bertil (9 September 2011). "Bertil Hille". In Squire, Larry R. (ed.). The History of Neuroscience in Autobiography: Volume 7. Oxford University Press. ISBN 978-0-19-990976-6 . Retrieved 10 May 2022.
- ↑ Hille, Bertil (9 May 2022). "A Life of Biophysics". Annual Review of Biophysics. 51 (1): 1–17. doi: 10.1146/annurev-biophys-120121-074034 . ISSN 1936-122X. PMID 34932910. S2CID 245397023.
- 1 2 Butler, Steve (28 June 2021). "Bertil Hille Continues as Professor Emeritus". The Huddle. Retrieved 10 May 2022.
- ↑ Kruger, Larisa C.; Isom, Lori L. (June 2016). "Voltage-Gated Na + Channels: Not Just for Conduction". Cold Spring Harbor Perspectives in Biology. 8 (6): a029264. doi:10.1101/cshperspect.a029264. PMC 4888818 . PMID 27252364.
- ↑ Hille, Bertil; Armstrong, Clay M.; MacKinnon, Roderick (October 1999). "Ion channels: From idea to reality" (PDF). Nature Medicine. 5 (10): 1105–1109. doi:10.1038/13415. PMID 10502800. S2CID 5216271 . Retrieved 10 May 2022.
- ↑ Brown, Angus M. (December 2019). "Ion channels: the concept emerges". The Journal of Physiology. 597 (24): 5725–5729. doi: 10.1113/JP279059 . ISSN 0022-3751. PMID 31617592. S2CID 204739080.
- 1 2 3 "Bertil Hille". Gairdner Foundation. Retrieved 10 May 2022.
- ↑ Brown, David A. (6 January 2020). "Neurons, Receptors, and Channels". Annual Review of Pharmacology and Toxicology. 60 (1): 9–30. doi: 10.1146/annurev-pharmtox-010919-023755 . ISSN 0362-1642. PMID 31914894. S2CID 210120471.
- ↑ Hille, Bertil (2001). Ion channels of excitable membranes (3rd ed.). Sunderland, Mass.: Sinauer. ISBN 9780878933211.
- 1 2 Stevens, Charles F. (February 2002). "Defining the field of ion channels". Nature Neuroscience. 5 (2): 93. doi: 10.1038/nn0202-93 . ISSN 1546-1726. S2CID 2469334.
- ↑ Goldstein, Steve A. N. (2 November 2001). "All Grown up and Ready to Rumble" (PDF). Cell. 107 (3): 274–276. doi:10.1016/S0092-8674(01)00555-4. ISSN 0092-8674. S2CID 18149385 . Retrieved 10 May 2022.
- ↑ Buehler, Lukas K. (May 5, 2003). "Reviews: Ionic Channels of Excitable Membranes". What is Life. Retrieved 26 February 2010.
- ↑ "Bertil Hille". National Academy of Sciences. Retrieved 10 May 2022.
- ↑ Dietz, Claire (May 15, 2003). "Catterall selected for Bristol-Myers Squibb Award in neuroscience research". UW News. Retrieved 10 May 2022.
- ↑ "The Louisa Gross Horwitz Prize for Biology Or Biochemistry" . Retrieved 26 February 2010.
- ↑ Renzulli, Virgil (October 11, 1996). "Two Biophysicists Win Columbia's Horwitz Prize". Columbia University Record. Retrieved 26 February 2010.
- ↑ "Bertil Hille". American Academy of Arts & Sciences. Retrieved 10 May 2022.
- ↑ "Function and structure of ion channel 1999 Albert Lasker Basic Medical Research Award". LASKER FOUNDATION. 2009. Retrieved 26 February 2010.
- ↑ "6 Scientists Named as Winners of Lasker Awards". The New York Times. 28 September 1999. Retrieved 10 May 2022.
- ↑ "UW professor Dr. Bertil Hille named to Institute of Medicine". UW News. October 15, 2002. Retrieved 10 May 2022.
- ↑ "Rockefeller University to hold 50th commencement June 12". News. June 9, 2008. Retrieved 10 May 2022.
- ↑ "The Bard Lectureship – Department of Physiology". Department of Physiology at The Johns Hopkins University School of Medicine. Retrieved 10 May 2022.