
Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.

Beryllium fluoride is the inorganic compound with the formula Be F2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.

Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not soluble but reactive with all protic organic solvents. It is soluble and nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest ionic compound.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.
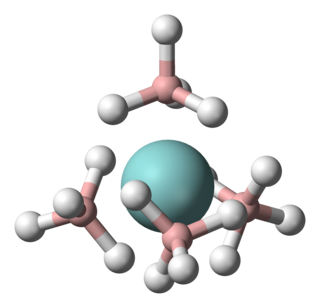
Uranium borohydride is the inorganic compound with the empirical formula U(BH4)4. Two polymeric forms are known, as well as a monomeric derivative that exists in the gas phase. Because the polymers convert to the gaseous form at mild temperatures, uranium borohydride once attracted much attention. It is solid green.

Borohydride refers to the anion [BH4]−, which is also called tetrahydridoborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing [BH4−nXn]−, where n is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate [BH3(CN)]− and triethylborohydride or triethylhydroborate [BH(CH2CH3)3]−. Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known. Tetrahydroborates are also of academic and industrial interest in inorganic chemistry.

Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).
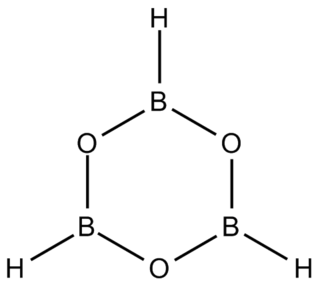
Boroxine is a 6-membered heterocyclic compound composed of alternating oxygen and singly-hydrogenated boron atoms. Boroxine derivatives such as trimethylboroxine and triphenylboroxine also make up a broader class of compounds called boroxines. These compounds are solids that are usually in equilibrium with their respective boronic acids at room temperature. Beside being used in theoretical studies, boroxine is primarily used in the production of optics.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).

Beryllium hydride is an inorganic compound with the chemical formula n. This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it. Unlike the ionically bonded hydrides of the heavier Group 2 elements, beryllium hydride is covalently bonded.
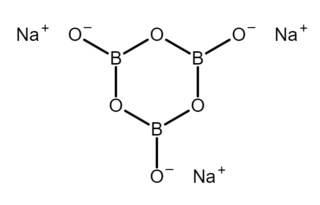
Sodium metaborate is a chemical compound of sodium, boron, and oxygen with formula NaBO2. However, the metaborate ion is trimeric in the anhydrous solid, therefore a more correct formula is Na3B3O6 or (Na+)3[B3O6]3−. The formula can be written also as Na2O·B2O3 to highlight the relation to the main oxides of sodium and boron. The name is also applied to several hydrates whose formulas can be written NaBO2·nH2O for various values of n.

Aluminium borohydride, also known as aluminium tetrahydroborate, is the chemical compound with the formula Al(BH4)3. It is a volatile pyrophoric liquid which is used as a reducing agent in laboratories. Unlike most other metal–borohydrides, which are ionic structures, aluminium borohydride is a covalent compound.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.
Cadmium hydride is an inorganic compound with the chemical formula (CdH
2)
n. It is a solid, known only as a thermally unstable, insoluble white powder.

Carbonyl hydrido tris(triphenylphosphine)rhodium(I) [Carbonyl(hydrido)tris(triphenylphosphane)rhodium(I)] is an organorhodium compound with the formula [RhH(CO)(PPh3)3] (Ph = C6H5). It is a yellow, benzene-soluble solid, which is used industrially for hydroformylation.

Organoberyllium chemistry involves the synthesis and properties of organometallic compounds featuring the group 2 alkaline earth metal beryllium (Be). The area remains understudied, relative to the chemistry of other main-group elements, because although metallic beryllium is relatively unreactive, its dust causes berylliosis and compounds are toxic. Organoberyllium compounds are typically prepared by transmetallation or alkylation of beryllium chloride.


















