Related Research Articles

Isoleucine is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, an α-carboxylic acid group, and a hydrocarbon side chain with a branch. It is classified as a non-polar, uncharged, branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria. It is encoded by the codons AUU, AUC, and AUA.
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excreted by cells to create non-cellular structures, such as hair, scales, feathers, or exoskeletons. Some nutrients can be metabolically converted to smaller molecules in the process of releasing energy, such as for carbohydrates, lipids, proteins, and fermentation products, leading to end-products of water and carbon dioxide. All organisms require water. Essential nutrients for animals are the energy sources, some of the amino acids that are combined to create proteins, a subset of fatty acids, vitamins and certain minerals. Plants require more diverse minerals absorbed through roots, plus carbon dioxide and oxygen absorbed through leaves. Fungi live on dead or living organic matter and meet nutrient needs from their host.
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine.
The net protein utilization, or NPU, is the ratio of amino acid mass converted to proteins to the mass of amino acids supplied. This figure is somewhat affected by the salvage of essential amino acids within the body, but is profoundly affected by the level of limiting amino acids within a foodstuff.

Vegetarian nutrition is the set of health-related challenges and advantages of vegetarian diets.

Whey protein is a mixture of proteins isolated from whey, the liquid material created as a by-product of cheese production. The proteins consist of α-lactalbumin, β-lactoglobulin, serum albumin and immunoglobulins. Whey protein is commonly marketed as a protein supplement, and various health claims have been attributed to it. A review published in 2010 in the European Food Safety Authority Journal concluded that the provided literature did not adequately support the proposed claims. For muscle growth, whey protein has been shown to be slightly better compared to other types of protein, such as casein or soy.
Protein digestibility-corrected amino acid score (PDCAAS) is a method of evaluating the quality of a protein based on both the amino acid requirements of humans and their ability to digest it.
Bodybuilding supplements are dietary supplements commonly used by those involved in bodybuilding, weightlifting, mixed martial arts, and athletics for the purpose of facilitating an increase in lean body mass. Bodybuilding supplements may contain ingredients that are advertised to increase a person's muscle, body weight, athletic performance, and decrease a person's percent body fat for desired muscle definition. Among the most widely used are high protein drinks, pre-workout blends, branched-chain amino acids (BCAA), glutamine, arginine, essential fatty acids, creatine, HMB, whey protein, ZMA, and weight loss products. Supplements are sold either as single ingredient preparations or in the form of "stacks" – proprietary blends of various supplements marketed as offering synergistic advantages.
Nitrogen balance is a measure of nitrogen input minus nitrogen output.
A complete protein or whole protein is a food source of protein that contains an adequate proportion of each of the nine essential amino acids necessary in the human diet.
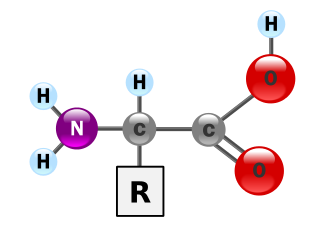
Proteins are essential nutrients for the human body. They are one of the building blocks of body tissue and can also serve as a fuel source. As a fuel, proteins provide as much energy density as carbohydrates: 4 kcal per gram; in contrast, lipids provide 9 kcal per gram. The most important aspect and defining characteristic of protein from a nutritional standpoint is its amino acid composition.
Protein combining or protein complementing is a dietary theory for protein nutrition that purports to optimize the biological value of protein intake. According to the theory, vegetarian and vegan diets may provide an insufficient amount of some essential amino acids, making protein combining with multiple foods necessary to obtain a complete protein food. The terms complete and incomplete are outdated in relation to plant protein. The position of the Academy of Nutrition and Dietetics is that protein from a variety of plant foods eaten during the course of a day supplies enough of all essential amino acids when caloric requirements are met.

Soy protein is a protein that is isolated from soybean. It is made from soybean meal that has been dehulled and defatted. Dehulled and defatted soybeans are processed into three kinds of high protein commercial products: soy flour, concentrates, and isolates. Soy protein isolate has been used since 1959 in foods for its functional properties.
Lactalbumin, also known as "whey protein", is the albumin contained in milk and obtained from whey. Lactalbumin is found in the milk of many mammals. There are alpha and beta lactalbumins; both are contained in milk.

Thomas Burr Osborne was a biochemist who, with Lafayette Mendel, independently discovered Vitamin A, though Elmer McCollum and Marguerite Davis were ultimately given credit, as they had submitted their paper first by three weeks. He is known for his work isolating and characterizing seed proteins, and for determining protein nutritional requirements. His career was spent at the Connecticut Agricultural Experiment Station.

Cecile Hoover Edwards was an American nutritional researcher whose career focused on improving the nutrition and well-being of disadvantaged people. Her scientific focus was on finding low-cost foods with an optimal amino acid composition, with a special interest in methionine metabolism. She was also a university administrator, serving as dean of several schools within Howard University between 1974 and 1990.

2016 was declared as the International Year of Pulses by the sixty eighth session of the United Nations General Assembly on December 20, 2013. The Food and Agriculture Organization (FAO) of the United Nations has been nominated to declare a year for pulses, more commonly known as legumes.
Protein quality is the digestibility and quantity of essential amino acids for providing the proteins in correct ratios for human consumption. There are various methods that rank the quality of different types of protein, some of which are outdated and no longer in use, or not considered as useful as they once were thought to be. The Protein Digestibility Corrected Amino Acid Score (PDCAAS), which was recommended by the Food and Agriculture Organization of the United Nations (FAO), became the industry standard in 1993. FAO has recently recommended the newer Digestible Indispensable Amino Acid Score (DIAAS) to supersede PDCAAS. The dairy industry is in favor of this, because while PDCAAS truncates all protein types that exceed the essential amino acid (EAA) requirements to 1.0, DIAAS allows a higher than 1.0 ranking: while for example both soy protein isolate and whey isolate are ranked 1.0 according to PDCAAS, in the DIAAS system, whey has a higher score than soy.

As in the human practice of veganism, vegan dog foods are those formulated with the exclusion of ingredients that contain or were processed with any part of an animal, or any animal byproduct. Vegan dog food may incorporate the use of fruits, vegetables, cereals, legumes including soya, nuts, vegetable oils, as well as any other non-animal based foods. The omnivorous domestic canine has evolved to metabolize carbohydrates and thrive on a diet lower in protein. A vegan diet is nutritionally adequate for dogs if properly formulated and balanced. Dogs can also thrive on a vegetarian diet.
Essential amino acids(EAAs) are amino acids that are necessary to build proteins in an organism. The source of complete EAAs are both animal and plant-based food.
References
- ↑ Thomas, K. Über die biologische Wertigkeit der stickstoff-substanzen in 1909 verschiedenen Nahrungsmitteln. Arch. Physiol., 219.
- ↑ Optimum Sports Nutrition: Your Competitive Edge, A Complete Nutritional Guide For Optimizing Athletic Performance; Chapter 12. by Dr. Michael Colgan
- ↑ The Great Animal Versus Vegetable Protein Debate What Is The Best Protein For Muscle Growth?
- 1 2 Mitchell, H.H. (1923). "A Method of Determining the Biological Value of Protein". Journal of Biol. Chem. 58 (3): 873.
- ↑ Chick H., Roscoe, M.H. (1930). "The biological values of proteins: A method for measuring the nitrogenous exchange of rats for the purpose of determining the biological value of proteins". Biochem J. 24 (6): 1780-2.
- ↑ Fixsen, M.A.B. "The biological value of purified caseinogen and the influence of vitamin B2 upon biological values, determined by the balance sheet method". Biochem J. 1930; 24(6): 1794–1804.
- ↑ S.G. Srikantia (August 1981). "The Use Of Biological Value Of A Protein In Evaluating Its Quality For Human Requirements". Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements Rome, 5 to 17 October 1981. Food and Agriculture Organization of the United Nations.
- ↑ Mitchell, H.H. A method for determining the biological value of protein. 1924 J. Biol. Chem., 58, 873. http://www.jbc.org/cgi/reprint/58/3/873.pdf
- ↑ Mitchell, H.H. and G.G. Carman. The biological value of the nitrogen of mixtures 1926 of patent white flour and animal foods. J. Biol. Chem., 68, 183.
- ↑ Recent developments in protein quality evaluation by Dr E. Boutrif.
- ↑ Hoffman, Jay R.; Falvo, Michael J. (2004). "Protein – Which is Best" (PDF). Journal of Sports Science and Medicine. 3 (3): 118–30. PMC 3905294 . PMID 24482589.
- 1 2 3 4 5 6 7 [Soybeans: Chemistry and Technology (copyright 1972) (b) Synder HE, Kwon TW. Soybean Utilization. Van Nostrand Reinhold Company, New York, 1987]
- 1 2 3 "ERRP | Expired Registration Recovery Policy". Archived from the original on 2016-03-04. Retrieved 2007-09-10.
- ↑ Eggum BO, Kreft I, Javornik B (1980). "Chemical-Composition and Protein-Quality of Buckwheat (Fagopyrum esculentum Moench)". Qualitas Plantarum Plant Foods for Human Nutrition. 30 (3–4): 175–9. doi:10.1007/BF01094020.
- 1 2 Jolliet, P. "Enteral nutrition in intensive care patients: a practical approach." Intensive Care Medicine (1998).
- ↑ Ruales J, Nair BM. "Nutritional quality of the protein in quinoa (Chenopodium quinoa, Willd) seeds." Plant Foods Hum Nutr. 1992 Jan;42(1):1-11.
- 1 2 3 "Microsoft PowerPoint - The Nutritious Egg" (PDF). Archived from the original (PDF) on 2007-08-07. Retrieved 2007-09-10.
- ↑ "Protein, Which Is Best" (PDF). JSSM . Retrieved 2007-10-31.
- ↑ Protein Quality-Report of Joint FAO’/WHO Expert Consultation, Food and Agriculture Organisation, Rome, FAO Food and Nutrition Paper 51, 1991.
- 1 2 3 4 "Biologische Wertigkeit Tabelle der besten Eiweiss Quellen -" (in German). 2012-06-01. Retrieved 2018-04-03.
- ↑ Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements, The use of biological value of protein in evaluatiing its quality for human requirements, S.G. Srikantia, University of Mysore.
- ↑ Testosterone Nation, The Protein Roundtable, August 24, 2000 Archived March 12, 2007, at the Wayback Machine .
- ↑ Journal of Sports Science and Medicine (2004) 3, 118-130
- ↑ Poullain, MG et al. Effect of whey proteins, their oligopeptide hydrosylates and free amino acid mixtures on growth and nitrogen retention in fed and starved rats. J Parenteral and Enteral Nutrition (1989) 13: 382-386
- 1 2 Pellett, PL and Young, VR. Nutritional evaluation of protein foods. United Nations University, 1980.
- ↑ The Use Of Biological Value Of A Protein In Evaluating Its Quality For Human Requirements.
- ↑ Said, A.K. and Hegsted, D.M., J. Nutr., 99, 474, 1969
- ↑ FAO/WHO (1991) Protein Quality Evaluation Report of Joint FAO/WHO Expert Consultation, Food and Agriculture Organization of the United Nations, FAO Food and Nutrition Paper No. 51, Rome.
- ↑ Schaafsma, G. (2000) 'The protein digestibility-corrected amino acid score. Journal of Nutrition 130, 1865S-1867S