
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".
In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrode (SHE). It is defined to have a potential of zero volts. It may also be defined as the potential difference between the charged metallic rods and salt solution.

Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.

A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.
In electrochemistry, a half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electric charges between the electrode and the electrolyte, resulting in a potential difference between the electrode and the electrolyte. The typical anode reaction involves a metal atom in the electrode being dissolved and transported as a positive ion across the double layer, causing the electrolyte to acquire a net positive charge while the electrode acquires a net negative charge. The growing potential difference creates an intense electric field within the double layer, and the potential rises in value until the field halts the net charge-pumping reactions. This self-limiting action occurs almost instantly in an isolated half-cell; in applications two dissimilar half-cells are appropriately connected to constitute a Galvanic cell.
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.

In electrochemistry, cyclic voltammetry (CV) is a type of potentiodynamic measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram, which plots the current produced by the analyte versus the potential of the working electrode.
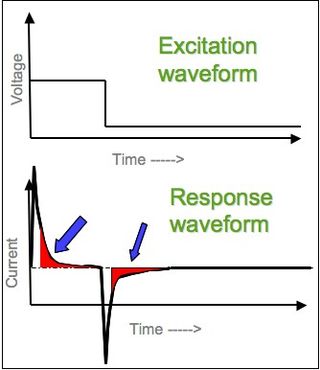
In electrochemistry, chronoamperometry is an analytical technique in which the electric potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells, this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio in comparison to other amperometric techniques.
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically-determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency. In an electrolytic cell the existence of overpotential implies that the cell requires more energy than thermodynamically expected to drive a reaction. In a galvanic cell the existence of overpotential means less energy is recovered than thermodynamics predicts. In each case the extra/missing energy is lost as heat. The quantity of overpotential is specific to each cell design and varies across cells and operational conditions, even for the same reaction. Overpotential is experimentally determined by measuring the potential at which a given current density is achieved.
In electrochemistry, electrosynthesis is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reactions, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.
Nanoarchitectures for lithium-ion batteries are attempts to employ nanotechnology to improve the design of lithium-ion batteries. Research in lithium-ion batteries focuses on improving energy density, power density, safety, durability and cost.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
In chemistry, ion transport number, also called the transference number, is the fraction of the total electric current carried in an electrolyte by a given ionic species i:
The Virtual breakdown mechanism is a concept in the field of electrochemistry. In electrochemical reactions, when the cathode and the anode are close enough to each other, the double layer of the regions from the two electrodes is overlapped, forming a large electric field uniformly distributed inside the entire electrode gap. Such high electric fields can significantly enhance the ion migration inside bulk solutions and thus increase the entire reaction rate, akin to the "breakdown" of the reactant(s). However, it is fundamentally different from the traditional "breakdown".
Electro-oxidation(EO or EOx), also known as anodic oxidation or electrochemical oxidation (EC), is a technique used for wastewater treatment, mainly for industrial effluents, and is a type of advanced oxidation process (AOP). The most general layout comprises two electrodes, operating as anode and cathode, connected to a power source. When an energy input and sufficient supporting electrolyte are provided to the system, strong oxidizing species are formed, which interact with the contaminants and degrade them. The refractory compounds are thus converted into reaction intermediates and, ultimately, into water and CO2 by complete mineralization.

Electrochemical quartz crystal microbalance (EQCM) is the combination of electrochemistry and quartz crystal microbalance, which was generated in the eighties. Typically, an EQCM device contains an electrochemical cells part and a QCM part. Two electrodes on both sides of the quartz crystal serve two purposes. Firstly, an alternating electric field is generated between the two electrodes for making up the oscillator. Secondly, the electrode contacting electrolyte is used as a working electrode (WE), together with a counter electrode (CE) and a reference electrode (RE), in the potentiostatic circuit constituting the electrochemistry cell. Thus, the working electrode of electrochemistry cell is the sensor of QCM.












