
Ferromagnetism is a property of certain materials that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagnetic materials are familiar metals that are noticeably attracted to a magnet, a consequence of their substantial magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an external magnetic field. This temporarily induced magnetization, for example, inside a steel plate, accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself depends not only on the strength of the applied field but on the so-called coercivity of the ferromagnetic material, which can vary greatly.

A magnet is a material or object that produces a magnetic field. This magnetic field is invisible but is responsible for the most notable property of a magnet: a force that pulls on other ferromagnetic materials, such as iron, steel, nickel, cobalt, etc. and attracts or repels other magnets.

In chemistry, thermodynamics, and other related fields, a phase transition is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point.
Remanence or remanent magnetization or residual magnetism is the magnetization left behind in a ferromagnetic material after an external magnetic field is removed. Colloquially, when a magnet is "magnetized", it has remanence. The remanence of magnetic materials provides the magnetic memory in magnetic storage devices, and is used as a source of information on the past Earth's magnetic field in paleomagnetism. The word remanence is from remanent + -ence, meaning "that which remains".
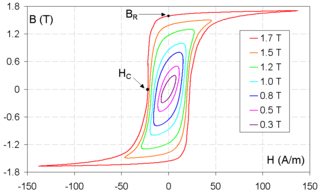
Coercivity, also called the magnetic coercivity, coercive field or coercive force, is a measure of the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized. Coercivity is usually measured in oersted or ampere/meter units and is denoted HC.

Alnico is a family of iron alloys which in addition to iron are composed primarily of aluminium (Al), nickel (Ni), and cobalt (Co), hence the acronym al-ni-co. They also include copper, and sometimes titanium. Alnico alloys are ferromagnetic, and are used to make permanent magnets. Before the development of rare-earth magnets in the 1970s, they were the strongest type of permanent magnet. Other trade names for alloys in this family are: Alni, Alcomax, Hycomax, Columax, and Ticonal.
Colossal magnetoresistance (CMR) is a property of some materials, mostly manganese-based perovskite oxides, that enables them to dramatically change their electrical resistance in the presence of a magnetic field. The magnetoresistance of conventional materials enables changes in resistance of up to 5%, but materials featuring CMR may demonstrate resistance changes by orders of magnitude.
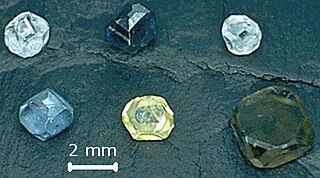
Imperfections in the crystal lattice of diamond are common. Such defects may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth. The defects affect the material properties of diamond and determine to which type a diamond is assigned; the most dramatic effects are on the diamond color and electrical conductivity, as explained by the electronic band structure.

A ferrite is a ceramic material made by mixing and firing iron(III) oxide with one or more additional metallic elements, such as strontium, barium, manganese, nickel, and zinc. They are ferrimagnetic, meaning they are attracted by magnetic fields and can be magnetized to become permanent magnets. Unlike other ferromagnetic materials, most ferrites are not electrically conductive, making them useful in applications like magnetic cores for transformers to suppress eddy currents. Ferrites can be divided into two families based on their resistance to being demagnetized.
Multiferroics are defined as materials that exhibit more than one of the primary ferroic properties in the same phase:
A quantum critical point is a point in the phase diagram of a material where a continuous phase transition takes place at absolute zero. A quantum critical point is typically achieved by a continuous suppression of a nonzero temperature phase transition to zero temperature by the application of a pressure, field, or through doping. Conventional phase transitions occur at nonzero temperature when the growth of random thermal fluctuations leads to a change in the physical state of a system. Condensed matter physics research over the past few decades has revealed a new class of phase transitions called quantum phase transitions which take place at absolute zero. In the absence of the thermal fluctuations which trigger conventional phase transitions, quantum phase transitions are driven by the zero point quantum fluctuations associated with Heisenberg's uncertainty principle.
The Reverse Monte Carlo (RMC) modelling method is a variation of the standard Metropolis–Hastings algorithm to solve an inverse problem whereby a model is adjusted until its parameters have the greatest consistency with experimental data. Inverse problems are found in many branches of science and mathematics, but this approach is probably best known for its applications in condensed matter physics and solid state chemistry.

Lanthanum strontium manganite (LSM or LSMO) is an oxide ceramic material with the general formula La1−xSrxMnO3, where x describes the doping level.
Gallium manganese arsenide, chemical formula (Ga,Mn)As is a magnetic semiconductor. It is based on the world's second most commonly used semiconductor, gallium arsenide,, and readily compatible with existing semiconductor technologies. Differently from other dilute magnetic semiconductors, such as the majority of those based on II-VI semiconductors, it is not paramagnetic but ferromagnetic, and hence exhibits hysteretic magnetization behavior. This memory effect is of importance for the creation of persistent devices. In (Ga,Mn)As, the manganese atoms provide a magnetic moment, and each also acts as an acceptor, making it a p-type material. The presence of carriers allows the material to be used for spin-polarized currents. In contrast, many other ferromagnetic magnetic semiconductors are strongly insulating and so do not possess free carriers. (Ga,Mn)As is therefore a candidate as a spintronic material.

Manganese(II) fluoride is the chemical compound composed of manganese and fluoride with the formula MnF2. It is a light pink solid, the light pink color being characteristic for manganese(II) compounds. It is made by treating manganese and diverse compounds of manganese(II) in hydrofluoric acid. Like some other metal difluorides, MnF2 crystallizes in the rutile structure, which features octahedral Mn centers.

The 122 iron arsenide unconventional superconductors are part of a new class of iron-based superconductors. They form in the tetragonal I4/mmm, ThCr2Si2 type, crystal structure. The shorthand name "122" comes from their stoichiometry; the 122s have the chemical formula AEFe2Pn2, where AE stands for alkaline earth metal (Ca, Ba, Sr or Eu) and Pn is pnictide (As, P, etc.). These materials become superconducting under pressure and also upon doping. The maximum superconducting transition temperature found to date is 38 K in the Ba0.6K0.4Fe2As2. The microscopic description of superconductivity in the 122s is yet unclear.
Heavy fermion superconductors are a type of unconventional superconductor.
Bismuth selenide is a gray compound of bismuth and selenium also known as bismuth(III) selenide.

Tetrataenite is a native metal alloy composed of chemically-ordered L10-type FeNi, recognized as a mineral in 1980. The mineral is named after its tetragonal crystal structure and its relation to the iron-nickel alloy, taenite. It is one of the mineral phases found in meteoric iron.
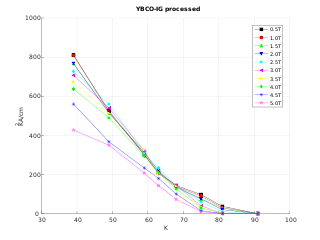
Rare-earth barium copper oxide (ReBCO) is a family of chemical compounds known for exhibiting high-temperature superconductivity (HTS). ReBCO superconductors have the potential to sustain stronger magnetic fields than other superconductor materials. Due to their high superconducting critical temperature and critical magnetic field, this class of materials are proposed for future use in technical applications where conventional low-temperature superconductors do not suffice. This includes magnetic confinement fusion reactors such as the ARC reactor, allowing a more compact and potentially more economical construction, and superconducting magnets to use in future particle accelerators to come after the Large Hadron Collider at CERN, which utilizes low-temperature superconductors.









