
Bismuth(III) oxide is a compound of bismuth, and a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite, but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the "Dragon's eggs" effect in fireworks, as a replacement of red lead.

Tritellurium dichloride is the inorganic compound with the formula Te3Cl2. It is one of the more stable lower chlorides of tellurium.

Bismuth chloride (or butter of bismuth) is an inorganic compound with the chemical formula BiCl3. It is a covalent compound and is the common source of the Bi3+ ion. In the gas phase and in the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory.

Bismuth oxychloride is an inorganic compound of bismuth with the formula BiOCl. It is a lustrous white solid used since antiquity, notably in ancient Egypt. Light wave interference from its plate-like structure gives a pearly iridescent light reflectivity similar to nacre. Previously, until the last decade of the twentieth century, bismuth oxochloride was known as bismuthyl chloride. It is also known as pigment pearl white.
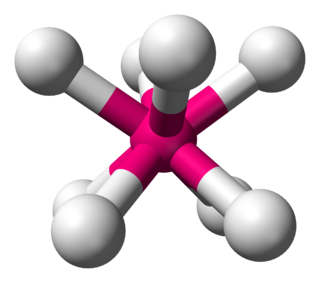
In chemistry, the square antiprismatic molecular geometry describes the shape of compounds where eight atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a square antiprism. This shape has D4d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the dodecahedron and the bicapped trigonal prism.

A localized surface plasmon (LSP) is the result of the confinement of a surface plasmon in a nanoparticle of size comparable to or smaller than the wavelength of light used to excite the plasmon. When a small spherical metallic nanoparticle is irradiated by light, the oscillating electric field causes the conduction electrons to oscillate coherently. When the electron cloud is displaced relative to its original position, a restoring force arises from Coulombic attraction between electrons and nuclei. This force causes the electron cloud to oscillate. The oscillation frequency is determined by the density of electrons, the effective electron mass, and the size and shape of the charge distribution. The LSP has two important effects: electric fields near the particle's surface are greatly enhanced and the particle's optical absorption has a maximum at the plasmon resonant frequency. Surface plasmon resonance can also be tuned based on the shape of the nanoparticle. The plasmon frequency can be related to the metal dielectric constant. The enhancement falls off quickly with distance from the surface and, for noble metal nanoparticles, the resonance occurs at visible wavelengths. Localized surface plasmon resonance creates brilliant colors in metal colloidal solutions.

Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitrate and bismuthyl nitrate. In older texts bismuth oxynitrate is often simply described as BiONO3 or basic bismuth nitrate. Bismuth oxynitrate was once called magisterium bismuti or bismutum subnitricum, and was used as a white pigment, in beauty care, and as a gentle disinfectant for internal and external use. It is also used to form Dragendorff's reagent, which is used as a TLC stain.

Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I).
The phosphidosilicates or phosphosilicides are inorganic compounds containing silicon bonded to phosphorus and one or more other kinds of elements. In the phosphosilicates each silicon atom is surrounded by four phosphorus atoms in a tetrahedron. The triphosphosilicates have a SiP3 unit, that can be a planar triangle like carbonate CO3. The phosphorus atoms can be shared to form different patterns e.g. [Si2P6]10− which forms pairs, and [Si3P7]3− which contains two-dimensional double layer sheets. [SiP4]8− with isolated tetrahedra, and [SiP2]2− with a three dimensional network with shared tetrahedron corners. SiP clusters can be joined, not only by sharing a P atom, but also by way of a P-P bond. This does not happen with nitridosilicates or plain silicates.
A selenite fluoride is a chemical compound or salt that contains fluoride and selenite anions. These are mixed anion compounds. Some have third anions, including nitrate, molybdate, oxalate, selenate, silicate and tellurate.
Gallium monoiodide is an inorganic gallium compound with the formula GaI or Ga4I4. It is a pale green solid and mixed valent gallium compound, which can contain gallium in the 0, +1, +2, and +3 oxidation states. It is used as a pathway for many gallium-based products. Unlike the gallium(I) halides first crystallographically characterized, gallium monoiodide has a more facile synthesis allowing a synthetic route to many low-valent gallium compounds.
The borophosphates are mixed anion compounds containing borate and phosphate anions, which may be joined together by a common oxygen atom. Compounds that contain water or hydroxy groups can also be included in the class of compounds.
Laser-induced white emission (LIWE) is a broadband light in the visible spectral range. This phenomenon was reported for the first time by Jiwei Wang and Peter Tanner in 2010 for fully concentrated lanthanide oxides in vacuum, excited by a focused beam of infrared laser diode operating in continuous wave (CW) mode. The white light emission intensity is exponentially dependent on excitation power density and pressure surrounding the samples. It was found that light emission is assisted by photocurrent generation and hot electron emission.
Selenogallates are chemical compounds which contain anionic units of selenium connected to gallium. They can be considered as gallates where selenium substitutes for oxygen. Similar compounds include the thiogallates and selenostannates. They are in the category of chalcogenotrielates or more broadly chalcogenometallates.
A Phosphide chloride is a mixed anion compound containing both phosphide (P3−) and chloride (Cl−) ions.
Phosphide iodides or iodide phosphides are compounds containing anions composed of iodide (I−) and phosphide (P3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the phosphide chlorides, arsenide iodides antimonide iodides and phosphide bromides.
Arsenide chlorides or chloride arsenides are compounds containing anions composed of chloride (Cl−) and arsenide (As3−). They can be considered as mixed anion compounds. They are in the category of pnictidehalides. Related compounds include the arsenide bromides, arsenide iodides, phosphide chlorides, and antimonide chlorides.

Bismuth forms mainly trivalent and a few pentavalent compounds. Many of its chemical properties are similar to those of arsenic and antimony, although much less toxic.
The stabilization of bismuth's +3 oxidation state due to the inert pair effect yields a plethora of organometallic bismuth-transition metal compounds and clusters with interesting electronics and 3D structures.

Caesium dibismuthide is an inorganic compound with the chemical formula CsBi2. It can obtained by reacting bismuth and caesium at 650 °C and allowing it to cool down, obtaining light silver crystals. The crystals belong to the cubic Fd3m space group, and have a Cu2Mg structure. Some sources point out that it is not sensitive to air and is slightly sensitive to moisture. However, other sources also mention that it decomposes and releases heat after being left in the air for a few minutes.
2. The Bi-Bi bond lengths are 3.07 A. EntryWithCollCode414090.png](http://upload.wikimedia.org/wikipedia/commons/thumb/6/65/EntryWithCollCode414090.png/220px-EntryWithCollCode414090.png)









