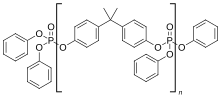
Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worked, molded, and thermoformed. Because of these properties, polycarbonates find many applications. Polycarbonates do not have a unique resin identification code (RIC) and are identified as "Other", 7 on the RIC list. Products made from polycarbonate can contain the precursor monomer bisphenol A (BPA).

A plasticizer is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture.
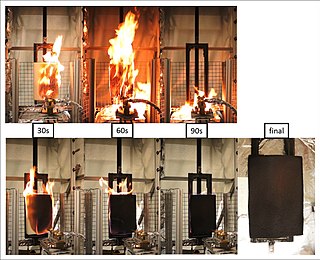
The term flame retardant subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and prevent or slow the further development of flames by a variety of different physical and chemical mechanisms. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or applied as a topical finish. Mineral flame retardants are typically additive, while organohalogen and organophosphorus compounds can be either reactive or additive.
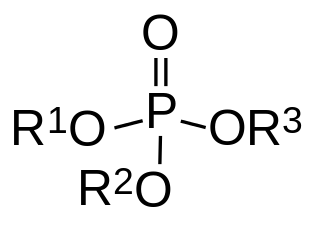
In organic chemistry, organophosphates are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid.
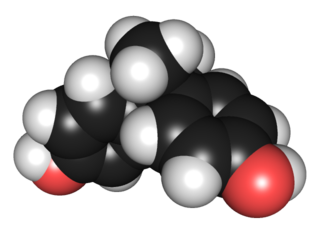
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.
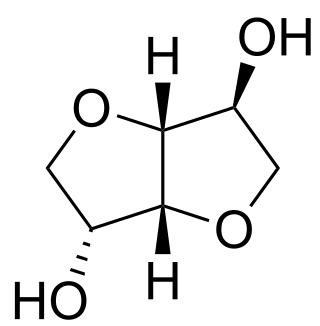
Isosorbide is a bicyclic chemical compound from the group of diols and the oxygen-containing heterocycles, containing two fused furan rings. The starting material for isosorbide is D-sorbitol, which is obtained by catalytic hydrogenation of D-glucose, which is in turn produced by hydrolysis of starch. Isosorbide is discussed as a plant-based platform chemical from which biodegradable derivatives of various functionality can be obtained.
Polysulfones are a family of high performance thermoplastics. These polymers are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl-SO2-aryl subunit. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Decabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). It was commercialised in the 1970s and was initially thought to be safe, but is now recognised as a hazardous and persistent pollutant. It was added to Annex A of the Stockholm Convention on Persistent Organic Pollutants in 2017, which means that treaty members must take measures to eliminate its production and use. The plastics industry started switching to decabromodiphenyl ethane as an alternative in the 1990s, but this is now also coming under regulatory pressure due to concerns over human health.
Pentabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).
Octabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs).

Triphenyl phosphate (TPhP) is the chemical compound with the formula OP(OC6H5)3. It is the simplest aromatic organophosphate. This colourless solid is the ester (triester) of phosphoric acid and phenol. It is used as a plasticizer and a fire retardant in a wide variety of settings and products.

Ammonium polyphosphate is an inorganic salt of polyphosphoric acid and ammonia containing both chains and possibly branching. Its chemical formula is H(NH4PO3)nOH showing that each monomer consists of an orthophosphate radical of a phosphorus atom with three oxygens and one negative charge neutralized by an ammonium cation leaving two bonds free to polymerize. In the branched cases some monomers are missing the ammonium anion and instead link to three other monomers.

Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless, low-melting solid. This, the simplest diaryl ether, has a variety of niche applications.

Diphenyl carbonate is the organic compound with the formula (C6H5O)2CO. It is classified as an acyclic carbonate ester. It is a colorless solid. It is both a monomer in combination with bisphenol A in the production of polycarbonate polymers and a product of the decomposition of polycarbonates.

High-performance plastics are plastics that meet higher requirements than standard or engineering plastics. They are more expensive and used in smaller amounts.

Bis(2-ethylhexyl)tetrabromophthalate (or TBPH), is a brominated phthalate derivative with the formula C24H34Br4O4 commonly used as a brominated flame retardant (BFR).