
Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean-Antoine-Claude Chaptal in 1790 when it was found that nitrogen was present in nitric acid and nitrates. Antoine Lavoisier suggested instead the name azote, from the Greek ἀζωτικός "no life", as it is an asphyxiant gas; this name is instead used in many languages, such as French, Italian, Russian, Romanian and Turkish, and appears in the English names of some nitrogen compounds such as hydrazine, azides and azo compounds.

Hydrazine is an inorganic compound with the chemical formula N
2H
4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour.
Unsymmetrical dimethylhydrazine (UDMH; 1,1-dimethylhydrazine ; heptyl) is a chemical compound with the formula H2NN(CH3)2 that is used as a rocket propellant. It is a colorless liquid, with a sharp, fishy, ammonia-like smell typical for organic amines. Samples turn yellowish on exposure to air and absorb oxygen and carbon dioxide. It is miscible with water, ethanol, and kerosene. In concentration between 2.5% and 95% in air, its vapors are flammable. It is not sensitive to shock. Symmetrical dimethylhydrazine, 1,2-dimethylhydrazine is also known but is not as useful.
Monomethylhydrazine (MMH) is a volatile hydrazine chemical with the chemical formula CH3(NH)NH2. It is used as a rocket propellant in bipropellant rocket engines because it is hypergolic with various oxidizers such as nitrogen tetroxide (N2O4) and nitric acid (HNO3). As a propellant, it is described in specification MIL-PRF-27404.
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. Originally reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912, it has been applied to the total synthesis of scopadulcic acid B, aspidospermidine and dysidiolide.
Morpholine is an organic chemical compound having the chemical formula O(CH2CH2)2NH. This heterocycle features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium. For example, treating morpholine with hydrochloric acid makes the salt morpholinium chloride. The naming of morpholine is attributed to Ludwig Knorr, who incorrectly believed it to be part of the structure of morphine.

Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2O2CN=NCO2CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanked by two ethyl ester groups. This orange-red liquid is a valuable reagent but also quite dangerous and explodes upon heating. Therefore, commercial shipment of pure diethyl azodicarboxylate is prohibited in the United States and is carried out either in solution or on polystyrene particles.
Hydrazides in organic chemistry are a class of organic compounds with the formula RNHNH2 where R is acyl (R'CO-), sulfonyl (R'SO2-), or phosphoryl (R'2P(O)-). Unlike hydrazine and alkylhydrazines, hydrazides are nonbasic owing to the inductive influence of the acyl, sulfonyl, or phosphoryl substituent.
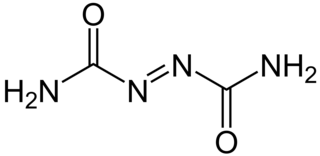
Azodicarbonamide, ADCA, ADA, or azo(bis)formamide, is a chemical compound with the molecular formula C2H4O2N4. It is a yellow to orange-red, odorless, crystalline powder. It is sometimes called a 'yoga mat' chemical because of its widespread use in foamed plastics. It was first described by John Bryden in 1959.

Iproniazid is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class. It is a xenobiotic that was originally designed to treat tuberculosis, but was later most prominently used as an antidepressant drug. However, it was withdrawn from the market because of its hepatotoxicity. The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in France until fairly recently.

Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes.

The Wharton olefin synthesis or the Wharton reaction is a chemical reaction that involves the reduction of α,β-epoxy ketones using hydrazine to give allylic alcohols. This reaction, introduced in 1961 by P. S. Wharton, is an extension of the Wolff–Kishner reduction. The general features of this synthesis are: 1) the epoxidation of α,β-unsaturated ketones is achieved usually in basic conditions using hydrogen peroxide solution in high yield; 2) the epoxy ketone is treated with 2–3 equivalents of a hydrazine hydrate in presence of substoichiometric amounts of acetic acid. This reaction occurs rapidly at room temperature with the evolution of nitrogen and the formation of an allylic alcohol. It can be used to synthesize carenol compounds. Wharton's initial procedure has been improved.

Hydrazines (R2N-NR2) are a class of chemical compounds which have two nitrogen atoms linked via a covalent bond and which carry from one up to four alkyl or aryl substituents. Hydrazines can be considered as derivatives of the inorganic hydrazine (H2N-NH2), in which one or more hydrogen atoms have been replaced by hydrocarbon groups.

Semicarbazide is the chemical compound with the formula OC(NH2)(N2H3). It is a water-soluble white solid. It is a derivative of urea.

Cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolone antibiotics. However the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner.
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, E (trans) and Z (cis). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene.

Sydnones are mesoionic heterocyclic chemical compounds possessing a 1,2,3-oxadiazole core with a keto group in the 5 position. Like other mesoionic compounds they are di-polar, possessing both positive and negative charges which are delocalized across the ring. Recent computational studies have indicated that sydnones and other similar mesoionic compounds are nonaromatic, "though well-stabilized in two separate regions by electron and charge delocalization." Sydnones are a heterocyclic compound named after the city of Sydney, Australia..

Cobalt tetracarbonyl hydride is an organometallic compound with the formula HCo(CO)4. It is a volatile, yellow liquid that forms a colorless vapor and has an intolerable odor. The compound readily decomposes upon melt and in absentia of high CO partial pressures forms Co2(CO)8. Despite operational challenges associated with its handling, the compound has received considerable attention for its ability to function as a catalyst in hydroformylation. In this respect, HCo(CO)4 and related derivatives have received significant academic interest for their ability to mediate a variety of carbonylation (introduction of CO into inorganic compounds) reactions.

An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application, oxaziridines are intermediates in the industrial production of hydrazine. Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions. Oxaziridines also serve as precursors to amides and participate in [3+2] cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Chiral oxaziridine derivatives effect asymmetric oxygen transfer to prochiral enolates as well as other substrates. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center.
The amino radical, •
NH
2, also known as the aminyl radical or azanyl radical, is the neutral form of the amide ion (NH−
2). Aminyl are highly reactive and consequently short lived like most radicals; however, they form an important part of nitrogen chemistry. In sufficiently high concentration, amino radicals dimerise to form hydrazine. While NH2 as a functional group is common in nature, forming a part of many compounds (e.g. the phenethylamines), the radical cannot be isolated in its free form.













