
Chlorine is a chemical element; it has symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

A gas mask is an item of personal protective equipment used to protect the wearer from inhaling airborne pollutants and toxic gases. The mask forms a sealed cover over the nose and mouth, but may also cover the eyes and other vulnerable soft tissues of the face. Most gas masks are also respirators, though the word gas mask is often used to refer to military equipment, the scope used in this article. The gas mask only protects the user from digesting, inhaling, and contact through the eyes. Most combined gas mask filters will last around 8 hours in a biological or chemical situation. Filters against specific chemical agents can last up to 20 hours.

The use of toxic chemicals as weapons dates back thousands of years, but the first large-scale use of chemical weapons was during World War I. They were primarily used to demoralize, injure, and kill entrenched defenders, against whom the indiscriminate and generally very slow-moving or static nature of gas clouds would be most effective. The types of weapons employed ranged from disabling chemicals, such as tear gas, to lethal agents like phosgene, chlorine, and mustard gas. This chemical warfare was a major component of the first global war and first total war of the 20th century. The killing capacity of gas was limited, with about 90,000 fatalities from a total of 1.3 million casualties caused by gas attacks. Gas was unlike most other weapons of the period because it was possible to develop countermeasures, such as gas masks. In the later stages of the war, as the use of gas increased, its overall effectiveness diminished. The widespread use of these agents of chemical warfare, and wartime advances in the composition of high explosives, gave rise to an occasionally expressed view of World War I as "the chemist's war" and also the era where weapons of mass destruction were created.

Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl. It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations and hypochlorite anions.
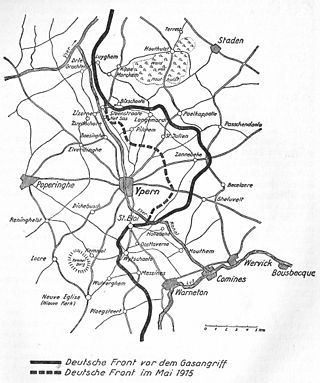
During the First World War, the Second Battle of Ypres was fought from 22 April – 25 May 1915 for control of the tactically important high ground to the east and south of the Flemish town of Ypres in western Belgium. The First Battle of Ypres had been fought the previous autumn. The Second Battle of Ypres was the first mass use by Germany of poison gas on the Western Front.

A respirator is a device designed to protect the wearer from inhaling hazardous atmospheres including fumes, vapours, gases and particulate matter such as dusts and airborne pathogens such as viruses. There are two main categories of respirators: the air-purifying respirator, in which respirable air is obtained by filtering a contaminated atmosphere, and the air-supplied respirator, in which an alternate supply of breathable air is delivered. Within each category, different techniques are employed to reduce or eliminate noxious airborne contaminants.

John Scott Haldane was a British physician physiologist and philosopher famous for intrepid self-experimentation which led to many important discoveries about the human body and the nature of gases. He also experimented on his son, the celebrated and polymathic biologist J. B. S. Haldane, even when he was quite young. Haldane locked himself in sealed chambers breathing potentially lethal cocktails of gases while recording their effect on his mind and body.

Colonel Cluny Macpherson was a physician and the inventor of an early gas mask. After World War I he served as the president of the St. John's Clinical Society and the Newfoundland Medical Association.

A smoke hood is a hood wherein a transparent airtight bag seals around the head of the wearer while an air filter held in the mouth connects to the outside atmosphere and is used to breathe. Smoke hoods are a class of emergency breathing apparatus intended to protect victims of fire from the effects of smoke inhalation. A smoke hood is a predecessor to the gas mask. The first modern smoke hood design was by Garrett Morgan and patented in 1912.

The P helmet, PH helmet and PHG helmet were early types of gas mask issued by the British Army in the First World War, to protect troops against chlorine, phosgene and tear gases. Rather than having a separate filter for removing the toxic chemicals, they consisted of a gas-permeable hood worn over the head which was treated with chemicals.
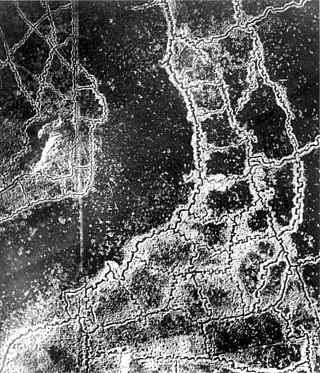
The Gas Attacks at Hulluch were two German cloud gas attacks on British troops during World War I, from 27 to 29 April 1916, near the village of Hulluch, 1 mi (1.6 km) north of Loos in northern France. The gas attacks were part of an engagement between divisions of the II Royal Bavarian Corps and divisions of the British I Corps.

Escape breathing apparatus, also called escape respirators, escape sets, self-rescuer masks, emergency life saving apparatus (ELSA), and emergency escape breathing devices (EEBD), are portable breathing apparatus that provide the wearer with respiratory protection for a limited period, intended for escape from or through an environment where there is no breathable ambient atmosphere. This includes escape through water and in areas containing harmful gases or fumes or other atmospheres immediately dangerous to life or health (IDLH).

The Hypo helmet, or British Smoke Hood, was an early British World War I gas mask, designed by Cluny Macpherson.

James Bert Garner was an American chemical engineer and professor at the Mellon Institute of Industrial Research from 1914 until his retirement in 1957. He is credited with the invention of a World War I gas mask design in 1915.

The M2 gas mask was a French-made gas mask used by French, British and American forces from April 1916 to August 1918 during World War I. The M2 was fabricated in large quantities, with about 29,300,000 being made during the war. It was intended to protect the wearer from at least five hours' exposure to phosgene gas, a common chemical weapon of the time.

The German phosgene attack of 19 December 1915 was the first use of phosgene gas against British troops by the German army. The gas attack took place at Wieltje, north-east of Ypres in Belgian Flanders on the Western Front in the First World War. German gas attacks on Allied troops had begun on 22 April 1915, during the Second Battle of Ypres using chlorine against French and Canadian units. The surprise led to the capture of much of the Ypres Salient, after which the effectiveness of gas as a weapon diminished, because the French and British introduced anti-gas measures and protective helmets. The German Nernst-Duisberg-Commission investigated the feasibility of adding the much more lethal phosgene to chlorine. Mixed chlorine and phosgene gas was used at the end of May 1915 against French troops and on Russian troops on the Eastern Front.

The Gas attacks at Wulverghem were German cloud gas releases during the First World War on British troops at Wulverghem in the municipality of Heuvelland, near Ypres in the Belgian province of West Flanders. The gas attacks were part of the sporadic fighting between battles in the Ypres Salient on the Western Front. The British Second Army held the ground from Messines Ridge northwards to Steenstraat and the divisions opposite the German XXIII Reserve Corps had received warnings of a gas attack. From 21 to 23 April, British artillery-fire exploded several gas cylinders in the German lines around Spanbroekmolen, which released greenish-yellow clouds. A gas alert was given on 25 April when the wind began to blow from the north-east and routine work was suspended; on 29 April, two German soldiers deserted and warned that an attack was imminent. Just after midnight on 30 April, the German attack began and over no man's land, a gas cloud drifted on the wind into the British defences, then south-west towards Bailleul.

A respirator fit test checks whether a respirator properly fits the face of someone who wears it. The fitting characteristic of a respirator is the ability of the mask to separate a worker's respiratory system from ambient air.
Chemical weapons have been a part of warfare in most societies for centuries. However, their usage has been extremely controversial since the 20th century.

The Small Box Respirator (SBC) was a British gas mask of the First World War and a successor to the Large Box Respirator. In late 1916, the respirator was introduced by the British with the aim to provide reliable protection against chlorine and phosgene gases. The respirator offered a first line of defence against these. The use of mustard gas, was begun by the Germans; a vesicant ("blister agent") that burnt the skin of individuals that were exposed to it. Death rates were high with exposure to both the mixed phosgene, chlorine and mustard gas, however with soldiers having readily available access to the small box respirator, death rates had lowered significantly. Light and reasonably fitting, the respirator was a key piece of equipment to protect soldiers on the battlefield.



















