In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood.

Chloral hydrate is a geminal diol with the formula C2H3Cl3O2. It is a colorless solid. It has limited use as a sedative and hypnotic pharmaceutical drug. It is also a useful laboratory chemical reagent and precursor. It is derived from chloral (trichloroacetaldehyde) by the addition of one equivalent of water.
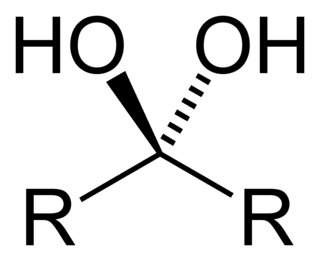
A geminal diol is any organic compound having two hydroxyl functional groups (-OH) bound to the same carbon atom. Geminal diols are a subclass of the diols, which in turn are a special class of alcohols. Most of the geminal diols are considered unstable.
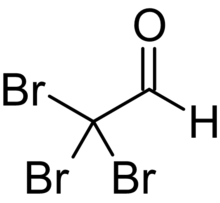
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.
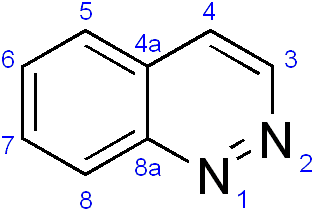
Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline.
Dichloralphenazone is a 1:2 mixture of antipyrine with chloral hydrate. In combination with paracetamol and isometheptene, it is the active ingredient of medications for migraine and tension headaches, including Epidrin and Midrin. Performance impairments are common with this drug and caution is advised, for example when driving motor vehicles. Additional uses of dichloralphenazone include sedation for the treatment of short-term insomnia, although there are probably better drug choices for the treatment of insomnia.
Melzer's reagent is a chemical reagent used by mycologists to assist with the identification of fungi, and by phytopathologists for fungi that are plant pathogens.
Chloral hydrate/magnesium sulfate/pentobarbital sodium, brand name Equithesin, is a combination anesthetic agent used as a general anesthetic in horses. It is administered intravenously to effect. For many years, it was the most commonly used injectable anesthetic in horses. Newer anesthetic agents such as injectable barbiturates, alpha-2 agonists, cyclohexylamines, and inhalants gradually replaced Equithesin. The drug has been off the market and unavailable for decades.
Chlorobutanol (trichloro-2-methyl-2-propanol) is an organic compound with the formula CCl3C(OH)(CH3)2. The compound is an example of a chlorohydrin. The compound is a preservative, sedative, hypnotic and weak local anesthetic similar in nature to chloral hydrate. It has antibacterial and antifungal properties. Chlorobutanol is typically used at a concentration of 0.5% where it lends long term stability to multi-ingredient formulations. However, it retains antimicrobial activity at 0.05% in water. Chlorobutanol has been used in anesthesia and euthanasia of invertebrates and fishes. It is a white, volatile solid with a camphor-like odor.

Matthias Eugen Oscar Liebreich was a German pharmacologist.
Acetylglycinamide chloral hydrate is a hypnotic/sedative. It is a combination of acetylglycinamide and chloral hydrate.

Petrichloral is a sedative and hypnotic chloral hydrate prodrug. It is a Schedule IV drug in the USA.

Amfecloral (INN), also known as amphecloral (USAN), is a stimulant drug of the phenethylamine and amphetamine chemical classes that was used as an appetite suppressant under the trade name Acutran, but is now no longer marketed. It was classified as an anorectic drug with little to no stimulant activity in a 1970 review. The British Pharmacopoeia Commission approved the name in 1970. The raw ingredients used in manufacturing it were d-amphetamine and chloral hydrate.

Chloral betaine, also known as cloral betaine (INN), is a sedative-hypnotic drug. It was introduced by Mead Johnson in the United States in 1963. It is a betaine complex with chloral hydrate, which acts as an extended-acting formulation of chloral hydrate which is then metabolized into trichloroethanol, which is responsible for most or all of its effects.

2,2,2-Tribromoethanol, often called just tribromoethanol, is a chemical compound with formula Br3C−CH2OH. Its molecule can be described as that of ethanol, with the three hydrogen atoms in position 2 replaced by bromine. It is a white crystalline solid, soluble in water and other solvents, that absorbs strongly in the UV below 290 nm.

A disulfiram-like drug is a drug that causes an adverse reaction to alcohol leading to nausea, vomiting, flushing, dizziness, throbbing headache, chest and abdominal discomfort, and general hangover-like symptoms among others. These effects are caused by accumulation of acetaldehyde, a major but toxic metabolite of alcohol formed by the enzyme alcohol dehydrogenase. The reaction has been variously termed a disulfiram-like reaction, alcohol intolerance, and acetaldehyde syndrome.

Sodium deuteroxide or deuterated sodium hydroxide is a chemical compound with the formula NaOD. It is a white solid very similar to sodium hydroxide, of which it is an isotopologue. It is used as a strong base and deuterium source in the production of other deuterated compounds. For example, reaction with chloral hydrate gives deuterated chloroform, and reaction with n-nitrosodimethylamine gives the deuterated analog of that compound.

Chloral cyanohydrin is the cyanohydrin derivative of chloral (trichloroacetaldehyde). It was historically used as a source of hydrogen cyanide for medicinal purposes. Chloral cyanohydrin is toxic by inhalation.
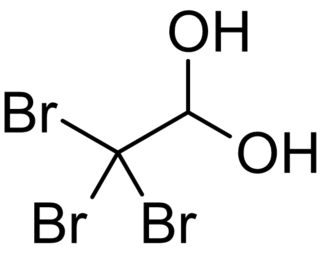
Bromal hydrate is an organobromine compound with the chemical formula C2H3Br3O2. It is the bromine analogue of chloral hydrate. Bromal hydrate forms when bromal is reacted with water. It decomposes to bromal and water upon distillation. It has hypnotic and analgesic properties but acts like a stimulant at lower doses. Bromal hydrate is more physiologically active than its chlorine analogue, chloral hydrate. Its direct effect on the heart muscles is stronger than that of chloral hydrate. Its analgesic effects were attributed to the proposed metabolism to bromoform.

Iodal, or triiodoacetaldehyde, is a halogenated derivative of acetaldehyde with the chemical formula I3CCHO, it is analogous to chloral and bromal. It is described as a pale yellow liquid with a pungent odour by Leopold Gmelin. It is decomposed to iodoform by potash. Iodal was discovered and named in 1837.