
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol, which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula CnH2n+1OH. Simple monoalcohols that are the subject of this article include primary, secondary and tertiary alcohols.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, an ester is a compound derived from an oxoacid in which at least one hydroxyl group is replaced by an alkoxy group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

Procaine is a local anesthetic drug of the amino ester group. It is most commonly used in dental procedures to numb the area around a tooth and is also used to reduce the pain of intramuscular injection of penicillin. Owing to the ubiquity of the trade name Novocain or Novocaine, in some regions, procaine is referred to generically as novocaine. It acts mainly as a sodium channel blocker. Today it is used therapeutically in some countries due to its sympatholytic, anti-inflammatory, perfusion-enhancing, and mood-enhancing effects.
4-Aminobenzoic acid (also known as para-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the para position) is an organic compound with the formula H2NC6H4CO2H. PABA is a white solid, although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino and carboxyl groups. The compound occurs extensively in the natural world.

Fischer esterification or Fischer–Speier esterification is a special type of esterification by refluxing a carboxylic acid and an alcohol in the presence of an acid catalyst. The reaction was first described by Emil Fischer and Arthur Speier in 1895. Most carboxylic acids are suitable for the reaction, but the alcohol should generally be primary or secondary. Tertiary alcohols are prone to elimination. Contrary to common misconception found in organic chemistry textbooks, phenols can also be esterified to give good to near quantitative yield of products. Commonly used catalysts for a Fischer esterification include sulfuric acid, p-toluenesulfonic acid, and Lewis acids such as scandium(III) triflate. For more valuable or sensitive substrates other, milder procedures such as Steglich esterification are used. The reaction is often carried out without a solvent or in a non-polar solvent to facilitate the Dean-Stark method. Typical reaction times vary from 1–10 hours at temperatures of 60-110 °C.

2-Butanol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. Its structural isomers are 1-butanol. isobutanol, and tert-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-2-butanol and (S)-(+)-2-butanol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.

n-Butyl acetate is an organic compound with the formula CH3(CH2)3O2CCH3. A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as an industrial solvent

Benzyl butyl phthalate (BBP) is an organic compound historically used a plasticizer, but which has now been largely phased out due to health concerns. It is a phthalate ester of containing benzyl alcohol, and n-butanol tail groups. Like most phthalates, BBP is non-volatile and remains liquid over a wide range of temperatures. It was mostly used as a plasticizer for PVC, but was also a common plasticizer for PVCA and PVB.

sec-Butyl acetate, or s-butyl acetate, is an ester commonly used as a solvent in lacquers and enamels, where it is used in the production of acyclic polymers, vinyl resins, and nitrocellulose. It is a clear flammable liquid with a sweet smell.

The bioconversion of biomass to mixed alcohol fuels can be accomplished using the MixAlco process. Through bioconversion of biomass to a mixed alcohol fuel, more energy from the biomass will end up as liquid fuels than in converting biomass to ethanol by yeast fermentation.
3-Nitrobenzoic acid is an organic compound with the formula C6H4(NO2)CO2H. It is an aromatic compound and under standard conditions, it is an off-white solid. The two substituents are in a meta position with respect to each other, giving the alternative name of m-nitrobenzoic acid. This compound can be useful as it is a precursor to 3-aminobenzoic acid, which is used to prepare some dyes.
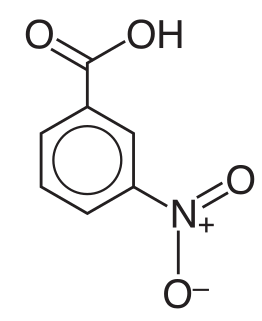
Nitrobenzoic acids are derivatives of benzoic acid. Two are commercially important. They are about ten times more acidic than the parent benzoic acid.
4-Nitrobenzoic acid is an organic compound with the formula C6H4(NO2)CO2H. It is a pale yellow solid. It is a precursor to 4-nitrobenzoyl chloride, the precursor to the anesthetic procaine and folic acid. It is also a precursor to 4-aminobenzoic acid.
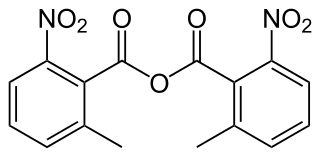
2-Methyl-6-nitrobenzoic anhydride is an organic acid anhydride also known as the Shiina reagent, having a structure wherein carboxylic acids undergo intermolecular dehydration condensation. It was developed in 2002 by Prof. Isamu Shiina. The compound is often abbreviated MNBA.
Shiina macrolactonization is an organic chemical reaction that synthesizes cyclic compounds by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic cyclization method using Lewis acid catalyst, and, in 2002, a basic cyclization using nucleophilic catalyst.
Butyl acrylate is an organic compound with the formula C4H9O2CCH=CH2. A colorless liquid, it is the butyl ester of acrylic acid. It is used commercially on a large scale as a precursor to polybutylacrylate, which is used in paints, sealants, coatings, adhesives, fuel, textiles, plastics, and caulk.
Shiina esterification is an organic chemical reaction that synthesizes carboxylic esters from nearly equal amounts of carboxylic acids and alcohols by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic coupling method using Lewis acid, and, in 2002, a basic esterification using nucleophilic catalyst.
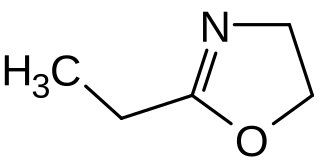
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s. This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.
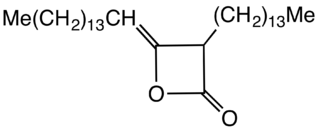
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position.















