Aldose reductase inhibitors are a class of drugs being studied as a way to prevent eye and nerve damage in people with diabetes.
Advanced glycation end products (AGEs) are proteins or lipids that become glycated as a result of exposure to sugars. They are a bio-marker implicated in aging and the development, or worsening, of many degenerative diseases, such as diabetes, atherosclerosis, chronic kidney disease, and Alzheimer's disease.
Aromatase inhibitors (AIs) are a class of drugs used in the treatment of breast cancer in postmenopausal women and in men, and gynecomastia in men. They may also be used off-label to reduce estrogen conversion when supplementing testosterone exogenously. They may also be used for chemoprevention in women at high risk for breast cancer.

Gingerol ([6]-gingerol) is a phenolic phytochemical compound found in fresh ginger that activates heat receptors on the tongue. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family and is high in concentrations in the grains of paradise as well as an African Ginger species.
Exemestane, sold under the brand name Aromasin among others, is a medication used to treat breast cancer. It is a member of the class of antiestrogens known as aromatase inhibitors. Some breast cancers require estrogen to grow. Those cancers have estrogen receptors (ERs), and are called ER-positive. They may also be called estrogen-responsive, hormonally-responsive, or hormone-receptor-positive. Aromatase is an enzyme that synthesizes estrogen. Aromatase inhibitors block the synthesis of estrogen. This lowers the estrogen level, and slows the growth of cancers.
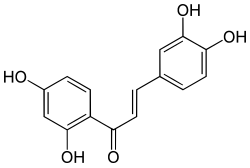
The polyol pathway is a two-step process that converts glucose to fructose. In this pathway glucose is reduced to sorbitol, which is subsequently oxidized to fructose. It is also called the sorbitol-aldose reductase pathway.

RAGE, also called AGER, is a 35 kilodalton transmembrane receptor of the immunoglobulin super family which was first characterized in 1992 by Neeper et al. Its name comes from its ability to bind advanced glycation endproducts (AGE), which include chiefly glycoproteins, the glycans of which have been modified non-enzymatically through the Maillard reaction. In view of its inflammatory function in innate immunity and its ability to detect a class of ligands through a common structural motif, RAGE is often referred to as a pattern recognition receptor. RAGE also has at least one other agonistic ligand: high mobility group protein B1 (HMGB1). HMGB1 is an intracellular DNA-binding protein important in chromatin remodeling which can be released by necrotic cells passively, and by active secretion from macrophages, natural killer cells, and dendritic cells.

Toxicodendron vernicifluum, also known by the common name Chinese lacquer tree, is an Asian tree species of genus Toxicodendron native to China and the Indian subcontinent, and cultivated in regions of China, Japan and Korea. Other common names include Japanese lacquer tree, Japanese sumac, and varnish tree. The trees are cultivated and tapped for their toxic sap, which is used as a highly durable lacquer to make Chinese, Japanese, and Korean lacquerware.
Hormonal therapy in oncology is hormone therapy for cancer and is one of the major modalities of medical oncology, others being cytotoxic chemotherapy and targeted therapy (biotherapeutics). It involves the manipulation of the endocrine system through exogenous or external administration of specific hormones, particularly steroid hormones, or drugs which inhibit the production or activity of such hormones. Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing, or even undergo cell death. Surgical removal of endocrine organs, such as orchiectomy and oophorectomy can also be employed as a form of hormonal therapy.
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism. This system has been studied in both bacteria and eukaryotes. This detoxification is accomplished by the sequential action of two thiol-dependent enzymes; firstly glyoxalase І, which catalyzes the isomerization of the spontaneously formed hemithioacetal adduct between glutathione and 2-oxoaldehydes into S-2-hydroxyacylglutathione. Secondly, glyoxalase ІІ hydrolyses these thiolesters and in the case of methylglyoxal catabolism, produces D-lactate and GSH from S-D-lactoyl-glutathione.

Diabetic cardiomyopathy is a disorder of the heart muscle in people with diabetes. It can lead to inability of the heart to circulate blood through the body effectively, a state known as heart failure(HF), with accumulation of fluid in the lungs or legs. Most heart failure in people with diabetes results from coronary artery disease, and diabetic cardiomyopathy is only said to exist if there is no coronary artery disease to explain the heart muscle disorder.

Fisetin (7,3′,4′-flavon-3-ol) is a plant flavonol from the flavonoid group of polyphenols. It can be found in many plants, where it serves as a yellow/ochre colouring agent. It is also found in many fruits and vegetables, such as strawberries, apples, persimmons, onions and cucumbers. Its chemical formula was first described by Austrian chemist Josef Herzig in 1891.
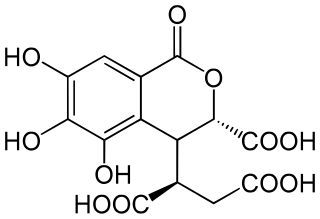
Chebulic acid is a phenolic compound isolated from the ripe fruits of Terminalia chebula.

Methaneseleninic acid is an organoselenium compound, a seleninic acid with the chemical formula CH3SeO2H. Its structure is CH3−Se(=O)−OH.

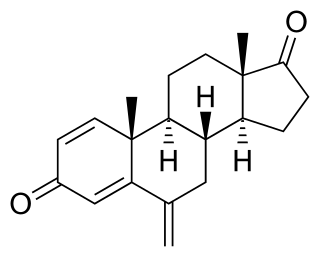
Palbociclib, sold under the brand name Ibrance among others, is a medication developed by Pfizer for the treatment of HR-positive and HER2-negative breast cancer. It is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6. Palbociclib was the first CDK4/6 inhibitor to be approved as a cancer therapy.
Steroidal aromatase inhibitors are a class of drugs that are mostly used for treating breast cancer in postmenopausal women. High levels of estrogen in breast tissue increases the risk of developing breast cancer and the enzyme aromatase is considered to be a good therapeutic target when treating breast cancer due to it being involved in the final step of estrogen biosynthetic pathway and also its inhibition will not affect production of other steroids. Aromatase Inhibitors are classified into two categories based on their structure, nonsteroidal and steroidal; the latter resemble the structure of androstenedione. Steroidal aromatase inhibitors irreversibly inhibit the enzyme by binding covalently to the binding site of aromatase so the substrate cannot access it.

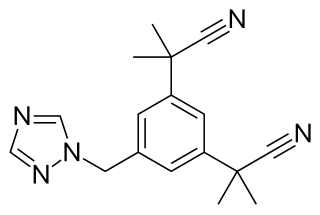
Norendoxifen, also known as 4-hydroxy-N,N-didesmethyltamoxifen, is a nonsteroidal aromatase inhibitor (AI) of the triphenylethylene group that was never marketed. It is an active metabolite of the selective estrogen receptor modulator (SERM) tamoxifen. Unlike tamoxifen, norendoxifen is not a SERM, and instead has been found to act as a potent and selective competitive inhibitor of aromatase (Ki = 35 nM). Drugs with dual SERM and AI activity, such as 4'-hydroxynorendoxifen, have been developed from norendoxifen, and may have therapeutic potential as antiestrogens in the treatment of estrogen receptor-positive breast cancer.

4'-Hydroxynorendoxifen is a synthetic, nonsteroidal antiestrogen of the triphenylethylene group. It is a dual selective estrogen receptor modulator (SERM) and aromatase inhibitor (AI), and was derived from tamoxifen, a SERM, and norendoxifen, a metabolite of tamoxifen that has been found to act as an AI. The drug has been suggested for potential development as a treatment for estrogen receptor (ER)-positive breast cancer. It was synthesized in 2015.

Non-Steroidal Aromatase Inhibitors (NSAIs) are one of two categories of aromatase inhibitors (AIs). AIs are divided into two categories, steroidal aromatase inhibitors and non-steroidal aromatase inhibitors that is based on their mechanism of action and structure. NSAIs are mainly used to treat breast cancer in women. NSAIs binding is a reversible process where NSAIs binds to the aromatase enzyme through non-covalent interactions. When aromatase inhibitors (AIs) are used to treat breast cancer the main target is the aromatase enzyme which is responsible for the high estrogen level.